Worldwide
Fresenius Helios
Europe
Largest European hospital operator
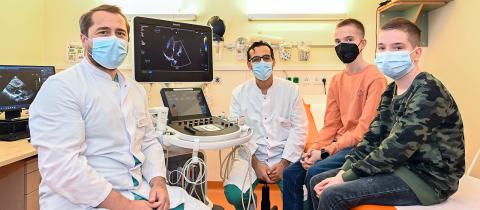
Fresenius Helios is Europe's leading private health care provider, with 127,000 employees. Together with Quirónsalud in Spain and Latin America, the Helios Group in Germany is part of the Helios Health holding. Every year about 26 million patients choose Helios for their medical treatment. In 2023, the company’s sales totaled more than €12 billion.Helios Germany operates more than 80 hospitals, about 230 outpatient care centers with 600 accredited doctor's licenses, six prevention centers and 27 occupational medicine centers. Helios treats approximately 5.5 million patients annually, of whom 4 million are outpatients. Since its beginnings, Helios has focused on measurable, high medical quality as well as data transparency and is better than the German average in 89 per cent of the quality targets. With more than 78,000 employees, Helios generated sales of €7.3 billion in 2023. Helios Germany is headquartered in Berlin.Quirónsalud operates 57 hospitals, including seven in Latin America, 100 outpatient centers and around 300 occupational risk prevention centers, and treats approximately 20 million patients annually, of whom 19 million are outpatients. Quirónsalud has more than 49,000 employees and generated sales of €4.8 billion in 2023.Helios is part of the Fresenius healthcare group.
Contact
Helios Kliniken GmbH
Friedrichstr. 136
10117 Berlin
Germany
T +49 30 521 321-0

Fresenius
Europe
Corporate Headquarters
The Fresenius corporate head office is located in Bad Homburg. Fresenius SE & Co. KGaA and the Fresenius Medical Care and Fresenius Kabi business segments have their main offices here.
Contact
Fresenius SE & Co. KGaA
Else-Kröner-Str. 1
61352 Bad Homburg
Germany
T: +49 6172 608-0
pr-fre@fresenius.com

Fresenius
Asia-Pacific
Successful in China for more than 30 years
China has played a major role in the development of Fresenius. The health care group was already active in “The Middle Kingdom” in the early 1980s, and has been gaining experience and know-how in this key market ever since. Fresenius Kabi now operates four plants in China. It is the market leader in parenteral nutrition, a leading supplier of infusion therapies, and one of the largest foreign-based pharmaceutical companies in the country. Fresenius Vamed has also successfully completed numerous projects in China. Among them: Equipping a number of hospitals with the latest medical technology, and erecting the 680-bed First People’s Hospital in Shanghai on a turnkey basis.
Contact
Fresenius Kabi (China) Co. Limited
16F, GrandyVic Building, No.16, Taiyanggong Mid Street
Chaoyang District
1000028 Beijing, China
T +86 10 5909 6999
Contact
Fresenius Medical Care (Shanghai) Co. Ltd.
Unit 4601-05&11, 46F, 2 Grand Gateway
3 Hongqiao Road, Xu Hui District
200030 Shanghai, China
T +86 21 6115 2800

Fresenius Helios
Europe
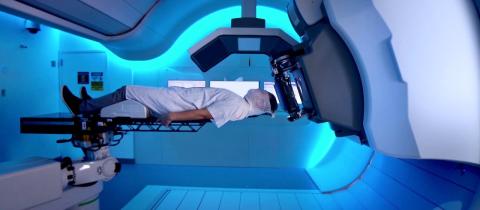
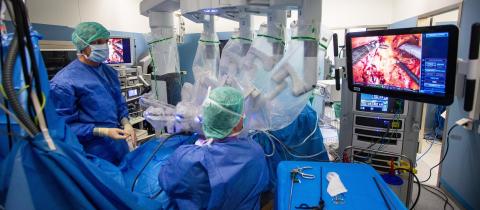
Using protons to take on cancer
One of the most advanced cancer treatments, proton beam therapy works by radiating the tumor with positively charged hydrogen atoms, and has major advantages over traditional radiation therapy. In 2020, Quirónsalud, the Spanish hospital group of Fresenius Helios, has opened that country’s first proton beam therapy center.Quirónsalud has opted for the Proteus One, a unique proton beam therapy system which, unlike others systems, allows all the technology necessary for treatment to be grouped together in one multifunctional room. It includes a tumor-scanning system to help doctors determine the optimal dose for each part of the body being treated, as well as a state-of-the-art imaging systems. And Proteus One can rotate 360 degrees, to direct the beam from any desired angle.
Fresenius Kabi
Europe
State of the art production of infusions
In Friedberg, Germany, Fresenius Kabi operates a state of the art production facility for infusion solutions. The plant employs about 1,000 people. The company delivers infusion solutions in plastic bags or bottles from Friedberg to pharma wholesalers, pharmacists and hospitals. Every day, the employees of the Friedberg Logistics Center send out around 2,000 orders with the aid of modern software and a fully-automated high-rack warehouse to customers all over the world.
Contact
Fresenius Kabi Deutschland GmbH
Werk Friedberg
Freseniusstr. 1
61169 Friedberg
Germany
T: +49 (0) 6172 686-0

Fresenius
Latin America
Major player in Latin America
Fresenius has been active in Brazil longer than in any other country outside of Europe, having set up its own distributor there in the mid-1970s. The acquisition of Hiplex, a pharmaceutical company specializing in infusion solutions and based in Campinas, Sao Paulo state, followed in 1977. Brazil continues to play a leading role in Fresenius’ Latin American activities. Fresenius Kabi and Fresenius Medical Care both have their own plants in the country, and Fresenius Medical Care also operates dialysis clinics. In total, Fresenius employs around 4,000 people in Brazil.
Contact
Fresenius Kabi Brasil Ltda.
Av. Marginal Projetada, 1652 - G1
06464-200 Tamboré - Barueri - São Paulo
Brazil
T +55 11 2504 1400