Fresenius is covered by the leading rating agencies Standard & Poor's, Moody's and Fitch. The following table shows Fresenius SE & Co. KGaA's current ratings.
Rating
| Standard & Poor's | Moody's | Fitch | |
|---|---|---|---|
Corporate Credit Rating | BBB | Baa3 | BBB- |
Outlook | stable | stable | stable |
Fresenius Capital Markets Day 2024
Our Fresenius Capital Markets Day 2024 took place on June 5, 2024 in London.
During the event, we presented and discussed Fresenius Helios’ role as Operating Company within #FutureFresenius, elaborated on its strategy in more detail and provided in-depth information on Fresenius Helios’ operating businesses and growth prospects.
Presenters were Michael Sen, CEO of Fresenius, and Robert Möller, CEO of Fresenius Helios, and his management team.
A recording of the Capital Markets Day is available below in various languages.
Webcast replay and downloads
Webcast replay and Downloads
Archive
The Fresenius Capital Markets Day took place in London on May 25, 2023, in a hybrid format. A replay of the webcast is available.
As part of the event, we presented and discussed Fresenius Kabi’s role as Operating Company within #FutureFresenius, elaborated on its “Vision 2026” in more detail and provided in-depth information on the company’s operating businesses and growth prospects.
Presenters were Michael Sen, CEO of Fresenius, and Pierluigi Antonelli, CEO of Fresenius Kabi, and his management team.
Contact

Senior Vice President Investor Relations
Head of Investor Relations
T: +49 (0) 6172 608-97033
nick.stone@fresenius.com
Financial Highlights FY/24
Group Revenue 1
€ 21526 m
+8% 2
FY/23: €20,307 m
Group EBIT 1
€ 2489 m
+10% 3
FY/23: €2,266 m
Net income 1, 4
€ 1461 m
+13% 3
FY/23: €1,300 m
EPS 1, 4
€ 2.59
+13% 3
FY/23: €2.31
KABI Revenue 1
€ 8414 m
+10% 2
FY/23: €8,009 m
HELIOS Revenue
€ 12739 m
+6% 1
FY/23: €11,952 m
1 Before special items
2 Organic growth rate adjusted for accounting effects related to Argentina hyperinflation
3 Growth rate at constant currency (cc) and adjusted for Argentina hyperinflation
4 Excluding Fresenius Medical Care
2024 – What a successful year!
Listen to CEO Michael Sen on how our #FutureFresenius transformation is clearly paying off: today, we are a simpler, more focused, and stronger healthcare company.
Letter to our shareholders
2024 was a dynamic and successful year for Fresenius. More than 176,000 colleagues have made Fresenius more innovative, more focused, and more efficient. This is an outstanding team effort, and millions of patients worldwide benefit from it.
Sustainability Statement
As a healthcare Group, we make a significant contribution to providing people with access to healthcare and producing the necessary medical technology and pharmaceuticals.
Fresenius Kabi Story
Throughout 2024, Fresenius Kabi has significantly strengthened its role as a leading provider of healthcare products and therapies for critically and chronically ill patients: by investing in key growth areas and making a difference in patients’ lives.
Fresenius Helios Story
Innovation in hospitals is key! Fresenius Helios is Europe’s leading private network of hospitals and outpatient clinics and is continuously expanding its role. Discover how they’re driving medical excellence and advancing digital solutions.
Library
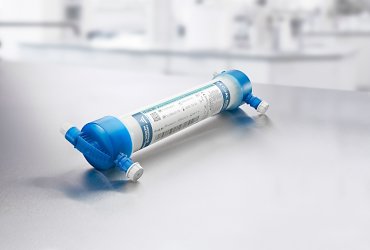
Fresenius Medical Care, the world's leading provider of products and services for individuals with renal diseases, is launching a new dialyzer for hemodialysis, the FX CorAL®. Focal points of the new dialyzer’s development were clinical performance and hemocompatibility. The FX CorAL is already available in Switzerland and will be gradually introduced in Germany, France, Italy and the United Arab Emirates. Other countries in the company’s Europe, Middle East and Africa region will follow.
Dialysis patients often suffer from chronic inflammation caused by the accumulation of toxic substances and the regular contact of their blood with external substances. These inflammations reduce patients’ quality of life and contribute to the onset of cardiovascular diseases. The core of the new FX CorAL is its new Helixone® hydro membrane, which forms a special gel-like layer of water on the surface of the inner membrane that reduces protein adsorption while the blood is being cleaned. This is to achieve a lower induction of the immune response in the patient while maintaining high selective permeability for the removal of toxins and excess water.
Dr. Olaf Schermeier, Fresenius Medical Care’s Chief Executive Officer for Global Research & Development, said: “The well-being of our patients is at the heart of everything we do at Fresenius Medical Care. Combining clinical performance with hemocompatibility is a central element of our patient centered dialysis: It was with precisely this goal that we developed the new FX CorAL.”
Dr. Katarzyna Mazur-Hofsäß, Chief Executive Officer for Fresenius Medical Care’s Europe, Middle East and Africa region, said: “We at Fresenius Medical Care are proud that every day we help to improve the lives of people with chronic kidney disease. We work continuously to further improve treatment quality for our patients. The launch of the new FX CorAL dialyzer in the EMEA region is another important step in this direction.”
The FX CorAL is the newest dialyzer in Fresenius Medical Care’s FX class of dialyzers, which use polysulfone, a special plastic with exceptional filtering and hemocompatibility characteristics. The special structure of the dialyzer’s fibers ensures that the dialysis fluid washes evenly around each fiber, improving clearance. A lateral blood inlet port prevents the bloodline from kinking during use, enabling a more homogenous blood flow and making treatment safer. In addition, all FX-class dialyzers are steam-sterilized and, owing to their light material, environmentally friendly. After receiving the CE-mark, the FX CorAL was introduced in Fresenius Medical Care’s NephroCare clinics and has already been used in more than 3 million treatments.
In hemodialysis, the dialyzer acts as an artificial kidney and takes over vital functions of the natural organ. Blood flows through as many as 20,000 extremely fine fibers, known as capillaries, which are clustered in a plastic tube approximately 30 centimeters (12 inches) long. Metabolic toxins and excess water are filtered from the blood through these capillaries, and then flushed out of the body with dialysis fluid. Blood cells and vital proteins, however, remain in the blood. This treatment is usually administered three times a week and lasts three to six hours per treatment.
About 43 percent of all dialyzers sold worldwide come from Fresenius Medical Care’s development and production sites.
Disclaimer:
This release contains forward-looking statements that are subject to various risks and uncertainties. Actual results could differ materially from those described in these forward-looking statements due to various factors, including, but not limited to, changes in business, economic and competitive conditions, legal changes, regulatory approvals, impacts related to the COVID-19 pandemic results of clinical studies, foreign exchange rate fluctuations, uncertainties in litigation or investigative proceedings, and the availability of financing. These and other risks and uncertainties are detailed in Fresenius Medical Care AG & Co. KGaA's reports filed with the U.S. Securities and Exchange Commission. Fresenius Medical Care AG & Co. KGaA does not undertake any responsibility to update the forward-looking statements in this release.
Signs marked with ® are registered trademarks of Fresenius Medical Care in selected countries.

May 13, 2022
Bad Homburg, Germany
Annual General Meeting 2022, Fresenius
Live-WebcastInformation, Documents and Shareholder Online Service
Quirónsalud, the largest private hospital group in Spain and part of Fresenius Helios, has signed agreements to acquire Centro Oncológico de Antioquia (COA) and Clínica Clofán, further expanding the company’s presence in Colombia. The clinics, located in Colombia's second largest city Medellín, will become part of Quirónsalud’s existing healthcare network in the country, which already comprises six hospitals and ten diagnostic centers.
COA is specialized in the diagnosis and treatment of cancer. It has 75 beds, four operating rooms, and specialized centers for nuclear medicine, radiotherapy and bone marrow transplants. Clínica Clofán is the second largest ophthalmic center in the city, with ten operating rooms and further dedicated facilities for treating even severe ophthalmic diseases and performing complex procedures.
Both clinics offer state-of-the-art medical standards and technology to their patients and are regarded as leading medical facilities with highly renowned physicians. Together they generate sales of around €30 million.
The acquisition is another important step in strengthening Fresenius Helios’ presence in the growing and consolidating healthcare services markets in Latin America.
The transaction is expected to close in the first quarter of 2022, pending anti-trust clearance of the Colombian authorities. Fresenius Helios expects the acquisition to be accretive to Fresenius’ Group net income already in fiscal year 2022.
This release contains forward-looking statements that are subject to various risks and uncertainties. Future results could differ materially from those described in these forward-looking statements due to certain factors, e.g. changes in business, economic and competitive conditions, regulatory reforms, results of clinical trials, foreign exchange rate fluctuations, uncertainties in litigation or investigative proceedings, and the availability of financing. Fresenius does not undertake any responsibility to update the forward-looking statements in this release.

Quirónsalud, the largest private hospital group in Spain and part of Fresenius Helios, has signed agreements to acquire Centro Oncológico de Antioquia (COA) and Clínica Clofán, further expanding the company’s presence in Colombia. The clinics, located in Colombia's second largest city Medellín, will become part of Quirónsalud’s existing healthcare network in the country, which already comprises six hospitals and ten diagnostic centers.
COA is specialized in the diagnosis and treatment of cancer. It has 75 beds, four operating rooms, and specialized centers for nuclear medicine, radiotherapy and bone marrow transplants. Clínica Clofán is the second largest ophthalmic center in the city, with ten operating rooms and further dedicated facilities for treating even severe ophthalmic diseases and performing complex procedures.
Both clinics offer state-of-the-art medical standards and technology to their patients and are regarded as leading medical facilities with highly renowned physicians. Together they generate sales of around €30 million.
The acquisition is another important step in strengthening Fresenius Helios’ presence in the growing and consolidating healthcare services markets in Latin America.
The transaction is expected to close in the first quarter of 2022, pending anti-trust clearance of the Colombian authorities. Fresenius Helios expects the acquisition to be accretive to Fresenius’ Group net income* already in fiscal year 2022.
*Net income attributable to shareholders of Fresenius SE & Co. KGaA
This release contains forward-looking statements that are subject to various risks and uncertainties. Future results could differ materially from those described in these forward-looking statements due to certain factors, e.g. changes in business, economic and competitive conditions, regulatory reforms, results of clinical trials, foreign exchange rate fluctuations, uncertainties in litigation or investigative proceedings, and the availability of financing. Fresenius does not undertake any responsibility to update the forward-looking statements in this release.
Fresenius Medical Care, the world’s leading provider of products and services for people with chronic kidney failure, has been recognized for the 12th time as a sustainability leader, with inclusion in the Dow Jones Sustainability Index. The DJSI Europe is composed of the top 20 percent of the largest 600 European companies listed in the S&P Global BMI, based on the international investment company S&P Global’s analysis of their economic, environmental and social performance. Fresenius Medical Care achieved improvements in most categories and was awarded maximum scores for partnerships toward sustainable healthcare, environmental reporting and social reporting. The company’s scores also rose significantly in the areas of risk management and customer relationship management.















