- Sales: € 8.4 billion, + 7 % at actual rates, + 11 % in constant currency
- EBIT: € 1.2 billion, + 12 % at actual rates, + 17 % in constant currency
- Net income: € 298 million, + 28 % at actual rates, + 32 % in constant currency
Outlook raised
Based on the Group's excellent financial results in the third quarter, Fresenius now expects net income to increase by more than 25 % in constant currency. Group sales are expected to grow by 9 to 10 % in constant currency. Previously, the Company expected net income to increase by ~25 % in constant currency and sales to grow by 8 to 10 % in constant currency.
Substantial growth: Group sales up 11 % in constant currency
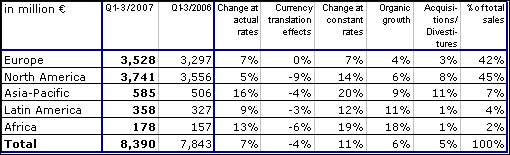
In the first three quarters Group sales increased by 11 % in constant currency and by 7 % at actual rates to € 8,390 million (Q1-3 2006: € 7,843 million). Organic sales growth was 6 %. Acquisitions contributed a further 6 %. Divestitures reduced sales growth by 1 %. Currency translation had a negative impact of 4 %.
In North America sales grew by 14 % in constant currency due to the Renal Care Group consolidation and an organic growth rate of 6 %. In Europe sales grew by 7 % in constant currency with organic sales growth contributing 4 %. Strong growth rates were achieved in the emerging markets with organic growth of 9 % in Asia-Pacific, 11 % in Latin America and 18 % in Africa.

Excellent earnings growth: net income up 32 % in constant currency
Group EBITDA increased by 15 % in constant currency and by 10 % at actual rates to € 1,485 million (Q1-3 2006: € 1,350 million). Group operating income (EBIT) grew by 17 % in constant currency and by 12 % at actual rates to € 1,184 million (Q1-3 2006: € 1,060 million; adjusted for the gain from the divestiture of US dialysis clinics and one-time expenses related to the Renal Care Group acquisition: € 1,036 million). The Group's EBIT margin improved to 14.1 % (Q1-3 2006: 13.5 %).
Group net interest was € -279 million (Q1-3 2006: € -295 million, including one-time expenses of € 30 million for the early refinancing of Group debt).
The tax rate was 36.0 % (Q1-3 2006: 40.9 %; adjusted for the tax expense related to the divestiture of US dialysis clinics: 37.8 %).
Minority interest increased to € 281 million (Q1-3 2006: € 219 million), of which 93 % was attributable to the minority interest in Fresenius Medical Care.
Group net income grew strongly by 32 % in constant currency and by 28 % at actual rates to € 298 million (Q1-3 2006: € 233 million, including one-time expenses of € 16 million).
Earnings per ordinary share were € 1.92 and earnings per preference share were € 1.93 (Q1-3 2006 adjusted for the February 2007 share split: ordinary share € 1.52, preference share € 1.53). This represents an increase of 26 % for both share classes.
Investments at a high level of € 727 million
Fresenius Group spent € 485 million for property, plant and equipment and intangible assets (Q1-3 2006: € 374 million). Acquisitions were € 242 million (Q1-3 2006: € 3,537 million).
Strong cash flow
Operating cash flow increased by 55 % to € 912 million (Q1-3 2006: € 588 million), driven by the strong earnings increase. In 2006, tax payments associated with the divestiture of US dialysis clinics had a negative effect. The cash flow margin was 10.9 % (Q1-3 2006: 7.5 %). Cash flow before acquisitions and dividends nearly doubled to € 447 million (Q1-3 2006: € 228 million). Free cash flow after acquisitions (€ 182 million) and dividends (€ 191 million) was € 74 million (Q1-3 2006: € -2,986 million).
Balance sheet structure: Net debt/EBITDA ratio improved
Fresenius Group's total assets increased by 4 % in constant currency and just slightly at actual rates to € 15,054 million (December 31, 2006: € 15,024 million). Current assets increased by 4 % to € 4,266 million (December 31, 2006: € 4,106 million). Non-current assets were € 10,788 million (December 31, 2006: € 10,918 million).
Shareholders' equity including minority interest grew by 4 % to € 5,946 million (December 31, 2006: € 5,728 million). The equity ratio (including minority interest) was 39.5 % (December 31, 2006: 38.1 %).
Group debt decreased by 5 % at actual rates and 1 % in constant currency to € 5,596 million (December 31, 2006: € 5,872 million). The net debt/EBITDA ratio improved to 2.7 as of September 30, 2007, well below the level of 3.0 as of December 31, 2006.
Number of employees increased
As of September 30, 2007, Fresenius increased the number of its employees by 5 % to 110,379 (December 31, 2006: 104,872).
Fresenius Biotech
Fresenius Biotech develops innovative therapies with trifunctional antibodies for the treatment of cancer as well as cell therapies for the treatment of the immune system. In the field of polyclonal antibodies, Fresenius Biotech has successfully marketed ATG-Fresenius S for many years. ATG-Fresenius S is an immunosuppressive agent used to prevent and treat graft rejection following organ transplantation.
After successful completion of the phase II/III study with removab® in patients with malignant ascites, submission for marketing authorization with the European Medicines Agency (EMEA) is expected for late 2007.
For the future marketing of removab in the USA and Japan, Fresenius Biotech is in discussions with potential partners.
Fresenius Biotech and Nabi Biopharmaceuticals agreed to terminate the agreement for the clinical development and marketing of ATG-Fresenius S in North America. Fresenius Biotech will assume responsibility for the clinical development and registration of ATG-Fresenius S in the US and continue with the ongoing phase III study.
In the first three quarters of 2007, Fresenius Biotech's operating income (EBIT) was € -33 million. For the full year 2007, Fresenius Biotech continues to expect EBIT of approximately € -50 million (2006: € -45 million).
The Business Segments
Fresenius Medical Care
Fresenius Medical Care is the world's leading provider of services and products for patients with chronic kidney failure. As of September 30, 2007, Fresenius Medical Care was serving 172,227 patients in 2,221 dialysis clinics.

- Strong organic sales growth of 7 %
- EBIT margin improved
- Earnings outlook at upper end expected
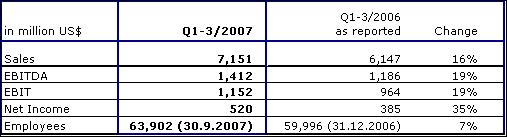
Fresenius Medical Care achieved strong sales growth of 16 % to US$ 7,151 million (Q1-3 2006: US$ 6,147 million). This was mainly driven by the strong organic growth of 7 % and by the consolidation of Renal Care Group (RCG). Sales in dialysis care increased by 16 % to US$ 5,357 million (Q1-3 2006: US$ 4,628 million). In dialysis products, sales grew by 18 % to US$ 1,794 million (Q1-3 2006: US$ 1,519 million).
In North America, sales growth was 14 % to US$ 4,957 million (Q1-3 2006: US$ 4,367 million). Sales outside North America ("International" segment) grew by 23 % (in constant currency: 15 %) to US$ 2,194 million (Q1-3 2006: US$ 1,780 million). This was driven by positive operating performance in Europe, the Asia-Pacific region and in Latin America.
EBIT rose by 19 % to US$ 1,152 million (Q1-3 2006: US$ 964 million; adjusted for the gain from the divestiture of US dialysis clinics and one-time expenses related to the RCG acquisition: US$ 936 million). The EBIT margin was 16.1 % (Q1-3 2006: 15.7 %; adjusted 15.2 %). Net income increased by 35 % to US$ 520 million (Q1-3 2006: US$ 385 million, including one-time expenses of US$ 20 million).
Fresenius Medical Care confirms its outlook for the full year 2007 and expects to achieve revenue of more than US$ 9.5 billion. This represents an increase of at least 12 %. Net income was projected to be in the range of US$ 685 million to US$ 705 million in 2007. Based on the strong performance in the third quarter, Fresenius Medical Care now expects the net income to be at the upper end of this guidance.
For further information, please see Fresenius Medical Care's press release at www.fmc-ag.com.
Fresenius Kabi
Fresenius Kabi offers infusion therapies and clinical nutrition for seriously and chronically ill patients in the hospital and out-patient environments. The company is also a leading provider of transfusion technology products.

- Strong organic sales growth of 7 %
- EBIT margin improves by 100 basis points to 16.2 %
- 2007 outlook fully confirmed
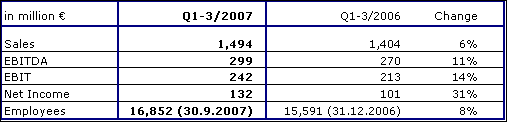
Fresenius Kabi increased sales by 6 % to € 1,494 million (Q1-3 2006: € 1,404 million). Currency translation effects had a negative impact of 2 %. This was mainly due to the depreciation of currencies in South Africa, China and Canada. Organic growth was 7 %. In the third quarter of 2007, Fresenius Kabi achieved an excellent organic growth of 8 %.
In Europe (excluding Germany) organic sales growth was 5 %. In Germany organic sales growth was 1 %. In the Asia-Pacific region Fresenius Kabi achieved significant organic sales growth of 22 %. Organic sales growth in Latin America was 10 % and in other regions 10 %.
Fresenius Kabi continued its excellent earnings growth. EBIT grew by 14 % to € 242 million (Q1-3 2006: € 213 million). The EBIT margin improved to 16.2 % (Q1-3 2006: 15.2 %). Fresenius Kabi reported strong growth in net income of 31 % to € 132 million (Q1-3 2006: € 101 million, including one-time expenses for early debt refinancing of € 11 million).
Fresenius Kabi fully confirms its outlook for the full year 2007. Organic sales growth is projected to be well into the 6 to 8 % range. Continued very strong sales growth is anticipated in particular from the regions outside Europe. Based on the positive sales projection and further manufacturing and logistics improvements Fresenius Kabi expects an EBIT margin of 16.0 to 16.5 % in 2007.
Fresenius ProServe
Fresenius ProServe is a leading German hospital operator with 58 facilities. Moreover, the company offers engineering and services for hospitals and other health care facilities.
- HELIOS achieves further operating margin improvement
- VAMED wins contract worth more than € 100 million
- Sales guidance for 2007 fully confirmed, EBIT guidance increased
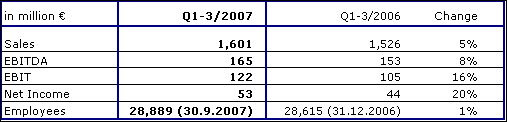
Fresenius ProServe achieved sales growth of 5 % to € 1,601 million (Q1-3 2006: € 1,526 million). Organic growth was 1 %, held back by delayed project revenues at VAMED. Acquisitions contributed 9 % whereas divestitures, primarily Pharmaplan and Pharmatec, had a negative impact of 5 %.
EBIT increased by 16 % to € 122 million (Q1-3 2006: € 105 million).
Sales in hospital operations (HELIOS Kliniken Group) increased by 12 % to € 1,348 million (Q1-3 2006: € 1,204 million). HELIOS achieved strong organic growth of 3 %. EBIT increased by 18 % to € 111 million (Q1-3 2006: € 94 million). The EBIT margin improved by 40 basis points to 8.2 %. In the third quarter of 2007, HELIOS reached an excellent EBIT margin of 9.4 % (Q3 2006: 8.7 %).
In July 2007, HELIOS agreed to acquire the Mariahilf hospital in Hamburg. The completion of the transaction is currently delayed by a legal proceeding against the seller of the hospital.
Sales in the engineering and services business were € 253 million (Q1-3 2006: € 322 million). The decrease is mainly due to the divestitures of Pharmaplan and Pharmatec, which were deconsolidated as of January 1, 2007, and June 30, 2007, respectively. Organic growth in the first three quarters was -7 % due to project delays at VAMED. EBIT was € 13 million (Q1-3 2006: € 14 million).
Order intake in the engineering business was € 245 million (Q1-3 2006: € 291 million). The decrease was due to the deconsolidation of the above-mentioned companies and the postponement of orders. In the third quarter of 2007, order intake increased by 31 % compared to the prior-year figure. This was mainly attributable to a contract worth more than € 100 million. The project includes the construction of a health and spa resort in Vienna/Austria. VAMED continues to expect an increase in its order intake in 2007 over 2006.
Due to its substantial order backlog of € 476 million (December 31, 2006: € 428 million) and its current view on the fourth quarter, VAMED remains confident to increase its sales in 2007 over 2006.
Based on the excellent financial results in 2007 to date, Fresenius ProServe raises its 2007 EBIT outlook from previously € ~170 million to more than € 170 million. The outlook for organic sales growth is confirmed at 2 to 3 %.
Press Conference
As part of the publication of the results for the first three quarters of 2007, a press conference will be held at the Fresenius headquarters in Bad Homburg on October 31, 2007 at 10 a.m. CET. All journalists are cordially invited to follow the conference in a live broadcast over out website at Press / Audio/Video Service. Following the meeting, a recording of the conference will be available as video-on-demand.
# # #
Fresenius is a health care group with international operations, providing products and services for dialysis, hospital and outpatient medical care. In 2006 group sales were approx. € 10.8 billion. On September 30, 2007 the Fresenius Group had 110,379 employees worldwide.
PDF-file includes:
Fresenius Group in Figures
- Consolidated statement of income (US GAAP) (unaudited)
- Key figures of the balance sheet (US GAAP) (unaudited)
- Cash flow statement (US GAAP) (unaudited)
- Segment reporting by business segment Q1-3 2007 (US GAAP) (unaudited)
During the American Society of Nephrology's Renal Week 2007 additional findings from the Calcium Acetate Renagel Evaluation (CARE 2) data set were presented by Dr. Wajeh Qunibi of the University of Texas Health Science Center, San Antonio. The study showed that there are no significant differences in aortic or mitral valve calcification between hemodialysis patients treated with PhosLo and those treated with selevamer if LDL is kept at constant levels. Previous findings from the same study have already shown that there is no difference in overall cardiovascular calcification in both treatment groups. The new findings also showed that the daily calcium intake from the use of calcium acetate as a phosphate binder for one year did not contribute to the progression of cardiovascular calcification in hemodialysis patients. These conclusions are consistent with the initial CARE-2 data presented at ASN renal week 2006.
PhosLo's safety and efficacy was further validated by the results of the Dialysis Clinical Outcomes Revisited (DCOR) trial, the first interventional outcomes study published in Kidney International, November 2007. The DCOR trial clearly demonstrated that there were no statistically significant differences in the all-cause or cardiovascular mortality among 2,100 hemodialysis patients randomized to sevelamer or calcium-based phosphate binders (CBPB). Additionally, a review of the laboratory data in DCOR demonstrated that the CBPB group had significantly better serum phosphorus and intact parathyroid hormone compared to the selevamer group (p<0.01).
These findings will provide additional data on the safety of PhosLo as the Company works with the Food and Drug Administration (FDA) to expand the PhosLo label indication to chronic kidney disease (CKD) predialysis. On October 16, 2007, the FDA's Cardiovascular and Renal Drugs Advisory Committee's recommended that the FDA extend the use of phosphate binders in predialysis patients with hyperphosphatemia. This favorable vote will allow the Company to work with the FDA on the regulatory pathway to this important label extension.
Dr. Ben Lipps, Chairman of the Management Board and Chief Executive Officer commented, "This data continues to validate our goal of providing safe and effective treatments to patients with bone and mineral abnormalities by offering pharmaceutical treatments as a part of our integrated "pharmatech" therapy."
About CARE-2
The CARE-2 study was a prospective, randomized, controlled head-to-head comparison between PhosLo and sevelamer with the addition of atorvastain calcium, as appropriate, in both treatment groups to control LDL (low density lipoprotein) levels. The study found not statistically significant difference in the progression of cardiovascular calcification (CAC) between the two treatment groups after 12 months of treatment. Patients treated with PhosLo and sevelamer achieved comparable reductions in serum phosphorus and calcium-phosphorus product and even more importantly K/DOQI target levels were reached significantly faster with PhosLo.
About PhosLo
PhosLo is a calcium acetate phosphate binder for oral application in renal disease patients. Excess phosphate consumed with food is normally removed by the kidneys in a process that can only partially be replaced by dialysis in patients with chronic kidney failure. Too much phosphate in the blood can result in a number of adverse events, including bone disease, thyroid problems and vascular calcification. The risk of such damage in end-stage renal disease patients can be lowered by regularly taking phosphate binders. Currently, the phosphate binder market exceeds $500 million worldwide. PhosLo is a trademark of Fresenius Medical Care.
# # #
Fresenius Medical Care is the world's largest integrated provider of products and services for individuals undergoing dialysis because of chronic kidney failure, a condition that affects more than 1,500,000 individuals worldwide. Through its network of 2,221 dialysis clinics in North America, Europe, Latin America, Asia-Pacific and Africa, Fresenius Medical Care provides dialysis treatment to 172,227 patients around the globe. Fresenius Medical Care is also the world's leading provider of dialysis products such as hemodialysis machines, dialyzers and related disposable products. Fresenius Medical Care is listed on the Frankfurt Stock Exchange (FME, FME3) and the New York Stock Exchange (FMS, FMS/P).
For more information about Fresenius Medical Care visit the Company's website at www.fmc-ag.com.
HELIOS Kliniken GmbH, a subsidiary of Fresenius SE, has agreed to acquire a majority stake in the Krefeld Municipal Hospitals. Yesterday, the Krefeld City Council voted to sell a 74.9 per cent interest in the hospitals to HELIOS. The Krefeld Municipal Hospitals include the Krefeld Clinic and the Cäcilien Hospital Krefeld-Hüls.
The Krefeld Clinic will be the fifth maximum-care hospital in the HELIOS clinic network following Berlin-Buch, Schwerin, Wuppertal and Erfurt. The Krefeld Clinic has a capacity of 1,023 beds and provided treatment for around 35,400 patients in 2006. The Cäcilien Hospital has 182 beds and treated about 4,600 patients. Both hospitals together have about 3,300 employees and achieved sales of approximately € 175 million in 2006.
The parties agreed not to disclose the purchase price. HELIOS will continue to develop the Krefeld Clinic as both a maximum-care and an academic teaching hospital and plans to strengthen the Cäcilien Hospital by expanding its medical services. HELIOS will construct a new hospital on the site of the Krefeld Clinic and plans to modernize the Cäcilien Hospital.
The acquisition is subject to the approval of the district and antitrust authorities. HELIOS expects to complete the transaction in the first half of 2008.
"The acquisition is one of the largest recent hospital privatizations and an important step in HELIOS' growth strategy," said Dr. Ulf M. Schneider, Chairman of the Management Board of Fresenius SE. "The hospitals provide an excellent geographic and medical fit to the HELIOS network. Krefeld's decision for HELIOS confirms our privatization concept which is focused on the highest levels of medical quality and transparency," he added.
HELIOS Kliniken Group operates 58 clinics of its own with around 16,000 beds, including four maximum-care hospitals in Erfurt, Berlin-Buch, Wuppertal and Schwerin. The company's 27,000 employees carry out 460,000 in-patient treatments per year. In 2006, the group achieved sales of € 1.7 billion.
# # #
Fresenius is a health care group with international operations, providing products and services for dialysis, hospital and outpatient medical care. In 2006 group sales were approx. € 10.8 billion. On September 30, 2007 the Fresenius Group had 110,379 employees worldwide.
Fresenius Kabi, a subsidiary of Fresenius SE, has reached an agreement to acquire Nestlé's enteral nutrition businesses in France (Novartis Nutrition S.A.S.) and in Spain (Nestlé Healthcare Nutrition Spain). With this acquisition, Fresenius Kabi strengthens its fast-growing segment Clinical Nutrition and significantly expands its market position in France and Spain.
Novartis Nutrition holds a leading position on the French enteral nutrition market and offers a comprehensive portfolio of sip and tube feeds and corresponding medical devices. With this acquisition, Fresenius Kabi will significantly improve its market position and will become the second largest provider of enteral nutrition products in France.
Nestlé Healthcare Nutrition Spain has successfully established itself as a renowned provider of enteral nutrition products in the Spanish market during the last few years. The acquisition will provide Fresenius Kabi with access to the Spanish enteral nutrition market.
In 2007, the businesses are projected to achieve combined sales of approximately € 55 million.
The European Commission made the divestiture of the businesses a condition in connection with Nestlé's acquisition of the worldwide clinical nutrition business of Novartis. Completion of the transaction was subject to both the approval of the European Commission and consultation and information of employees' representatives in France and Spain, all of which have now been satisfactorily completed.
The parties agreed not to disclose the purchase price.
The transaction is expected to close in 2007.
Clinical Nutrition:
In clinical nutrition Fresenius Kabi provides parenteral nutrition (administered intravenously) and enteral nutrition (administered as sip or tube feed via the gastrointestinal tract) as well as nutrition pumps and infusion disposables.
# # #
Fresenius is a health care group with international operations, providing products and services for dialysis, hospital and outpatient medical care. In 2006 group sales were approx. € 10.8 billion. On September 30, 2007 the Fresenius Group had 110,379 employees worldwide.
Fresenius Kabi is the leader in infusion therapy and clinical nutrition in Europe and in its most important countries of Latin America and Asia Pacific. Fresenius Kabi's core product range includes infusion solutions for fluid substitution, blood volume expansion and parenteral nutrition, as well as products for enteral nutrition. Furthermore the company provides concepts for ambulatory health care and is focused on managing and providing home therapies. With its philosophy "Caring for life" and a broad product portfolio, the company aims at improving the quality of life of patients all over the world. On September 30, 2007 the company had 16,852 employees. In 2006, Fresenius Kabi achieved sales of € 1,893 million and an operating profit of € 291 million. Fresenius Kabi AG is a 100% subsidiary of the health care group Fresenius SE.
Fresenius Medical Care AG & Co. KGaA today announced that it has acquired Renal Solutions, Inc. (RSI). The acquisition agreement provides for total consideration of up to $190 million, consisting of $100 million at closing, $60 million after the first year, and up to $30 million in milestone payments over the next three years. RSI had approximately $10 million of net debt outstanding at closing.
RSI is currently commercializing the Allient Sorbent Hemodialysis System, which is returning sorbent-based technology (SORB) to the dialysis field. The SORB cartridge has a long market history in hemodialysis with over 6 million cartridges sold. As the innovator in the SORB technology field, RSI holds key patents and other intellectual property worldwide related to the SORB technology.
The sorbent technology purifies tap water to dialysate quality and allows dialysate to be regenerated. This reduces the water volume requirement for a typical hemodialysis treatment from 120 liters / 37 gallons of reverse osmosis water to just 6 liters / 1.5 gallons of drinking water per treatment.
The combination of Fresenius Medical Care's leading hemodialysis technology and the SORB technology will provide a platform for superior home products and therapies. Furthermore, the significant reduction of dialysate through SORB technology is one major step towards miniaturization – a pre-requisite for the wearable kidney concept which could benefit certain patients and complement clinical-based therapy.
Fresenius Medical Care sees the current market size of the Home Therapy Market (Peritoneal Dialysis and Home Hemodialysis) at about $2 billion representing approximately 11% of the overall worldwide dialysis market. The Company believes the Home Therapy market has the potential to grow to $4 billion within the next 10 years. Fresenius Medical Care has a market share in this market segment of approximately 30%. Home hemodialysis (HHD) has been a niche market for many years but with growing attention in recent past. With increased access to adequate therapy, the Company projects the number of HHD patients in North America could grow from about 0.5% at the end of 2006 to approximately 4% of the patient population in the next 10 years.
Dr. Ben Lipps, Chief Executive Officer of Fresenius Medical Care commented, "The acquisition of RSI is an important step to advance the technology required for strong future growth in this field. The combination offers us the long-term opportunity to extend our leadership to home and acute dialysis products. Furthermore, by combining our equipment and membrane technology with the SORB technology, we can provide innovative solutions in the future such as a possible wearable kidney. With this acquisition, Fresenius Medical Care expects to increase its annual R&D spending by approximately $10 million starting in 2008. Our mid-term financial targets for the years 2007 through 2010 remain unchanged."
# # #
Fresenius Medical Care is the world's largest integrated provider of products and services for individuals undergoing dialysis because of chronic kidney failure, a condition that affects more than 1,500,000 individuals worldwide. Through its network of 2,221 dialysis clinics in North America, Europe, Latin America, Asia-Pacific and Africa, Fresenius Medical Care provides dialysis treatment to 172,227 patients around the globe. Fresenius Medical Care is also the world's leading provider of dialysis products such as hemodialysis machines, dialyzers and related disposable products. Fresenius Medical Care is listed on the Frankfurt Stock Exchange (FME, FME3) and the New York Stock Exchange (FMS, FMS/P).
For more information about Fresenius Medical Care visit the Company's website at www.fmc-ag.com.
For more information about Renal Solutions Inc. visit the Company's website at www.renalsolutionsinc.com.
Fresenius Kabi, a subsidiary of Fresenius SE, has reached an agreement to acquire the Chilean company Laboratorio Sanderson S.A. Sanderson is the Chilean market leader in intravenously administered generic drugs (I.V. drugs) and infusion solutions.
Fresenius Kabi already holds the leading market position in blood volume replacement solutions, intravenous anesthesia, and parenteral nutrition in Chile. Sanderson offers a high-quality range of antibiotics, analgesics, anesthetics and infusion solutions in Chile and other Latin American countries.
Through the acquisition Fresenius Kabi significantly expands its local product range and will become the leading infusion therapy provider in the Chilean hospital market. At the same time, Sanderson's product line, which is approved in Latin America, provides further excellent growth opportunities. The company's state-of-the-art production unit in Santiago de Chile facilitates Fresenius Kabi's product program rollout and its expansion into other Latin American countries.
Sanderson is privately-held and employs about 375 people in Chile and its subsidiary in Peru. It expects sales of € 19 million in 2007.
The acquisition is expected to close in January 2008.
# # #
Fresenius is a health care group with international operations, providing products and services for dialysis, hospital and outpatient medical care. In 2006 group sales were approx. € 10.8 billion. On September 30, 2007 the Fresenius Group had 110,379 employees worldwide.
Fresenius Kabi is the leader in infusion therapy and clinical nutrition in Europe and in its most important countries of Latin America and Asia Pacific. Fresenius Kabi's core product range includes infusion solutions for fluid substitution, blood volume expansion and parenteral nutrition, as well as products for enteral nutrition. Furthermore the company provides concepts for ambulatory health care and is focused on managing and providing home therapies. With its philosophy "Caring for life" and a broad product portfolio, the company aims at improving the quality of life of patients all over the world. On September 30, 2007 the company had 16,852 employees. In 2006, Fresenius Kabi achieved sales of € 1,893 million and an operating profit of € 291 million. Fresenius Kabi AG is a 100% subsidiary of the health care group Fresenius SE.
Fresenius Kabi, a subsidiary of Fresenius SE, has reached an agreement to acquire the Italian company Ribbon S.r.L. Ribbon is a leading European manufacturer of the antibiotic agent classes cephalosporines and penicillines with two state-of-the-art production facilities in northern Italy.
In the field of intravenously administered drugs (I.V. drugs) Fresenius Kabi offers, among others, a comprehensive range of products with the antibiotic agents cephalosporines and penicillines. With the acquisition of Ribbon, Fresenius Kabi becomes one of the few I.V. drug suppliers globally who have know-how and manufacturing expertise along the entire pharmaceutical value chain. The acquisition is a further step in the company's generic I.V. drug growth plan and significantly strengthens Fresenius Kabi's market position. At the same time, the company is ensuring its own supply of high-quality active agents for its antibiotic products.
Dr. Ulf M. Schneider, Chairman of the Management Board of Fresenius SE: "With the acquisition of Sanderson in Chile announced last week and today's acquisition of Ribbon we continue our Fresenius Kabi growth strategy. Sanderson provides an excellent platform for further expansion in Latin America. The Ribbon acquisition strengthens our position in the intravenously administered drug market and is an important step towards achieving quality and cost leadership in this product segment."
Ribbon is headquartered in Milan and has about 130 associates. The company is privately-held and expects sales of approximately € 54 million in 2007.
The transaction is expected to close in January 2008.
# # #
Fresenius is a health care group with international operations, providing products and services for dialysis, hospital and outpatient medical care. In 2006 group sales were approx. € 10.8 billion. On September 30, 2007 the Fresenius Group had 110,379 employees worldwide.
Fresenius Kabi is the leader in infusion therapy and clinical nutrition in Europe and in its most important countries of Latin America and Asia Pacific. Fresenius Kabi's core product range includes infusion solutions for fluid substitution, blood volume expansion and parenteral nutrition, as well as products for enteral nutrition. Furthermore the company provides concepts for ambulatory health care and is focused on managing and providing home therapies. With its philosophy "Caring for life" and a broad product portfolio, the company aims at improving the quality of life of patients all over the world. On September 30, 2007 the company had 16,852 employees. In 2006, Fresenius Kabi achieved sales of € 1,893 million and an operating profit of € 291 million. Fresenius Kabi AG is a 100% subsidiary of the health care group Fresenius SE.
Fresenius Biotech has dispatched the marketing authorization application for the trifunctional antibody Removab (INN: catumaxomab) in patients with malignant ascites to the European Medicines Agency (EMEA) as planned. The company applies for the EU authorization of Removab for the intraperitoneal treatment of malignant ascites in patients with epithelial cancers where no standard therapy is available or no longer feasible. The results of the phase II/III pivotal study announced in December 2006 as well as in March and July 2007 are an essential part of the marketing authorization application. In addition to the clinical results, the application contains preclinical data as well as production and product quality information. The scientific assessment of the marketing authorization application will start in early 2008 after the completion of the validation by EMEA.
Dr. Ulf M. Schneider, Chairman of the Management Board of Fresenius SE, commented: "The marketing authorization application for our Removab antibody is an important milestone for Fresenius Biotech. The results of the phase II/III pivotal study show clear benefits for patients treated with Removab. We believe that Removab could become a new therapy option for malignant ascites. We are encouraged to continue our Removab clinical trial program and will focus on applications in solid tumors."
Trifunctional Antibodies
Trifunctional antibodies are proteins that activate different cell types of the immune system simultaneously and direct these to the tumor cells by a targeted approach. Trifunctional antibodies therefore are very effective in destroying cancer cells and show a therapeutic effect even at very low doses. They are being developed by TRION Pharma GmbH.
Mode of action of trifunctional antibody removab (catumaxomab)
The therapeutic objective of trifunctional antibodies is to generate a stronger immune reaction against tumor cells. removab has two different antigen binding sites: While one arm of the antibody recognizes and binds to T-cells, the other arm binds EpCAM (epithelial cell adhesion molecule) that is overexpressed in many types of epithelial cancers. Immune effector cells with Fc receptors (macrophages, monocytes, dendritic cells and natural killer cells) can also bind the Fc region of intact trifunctional antibodies. This simultaneous binding subsequently results in the costimulation and activation of T-cells and accessory cells, enabling the generation of a strong immune response against tumor cells. Preclinical data also suggest a potential long-lasting effect to prevent cancer recurrence. Apart from removab two other trifunctional antibodies targeting other cancer antigens are currently undergoing clinical development.
Fresenius Biotech is a company within the Fresenius health care group and is focused on the development and marketing of biopharmaceuticals in the fields of oncology, immunology and regenerative medicine. For further information please visit www.fresenius-biotech.com.
Fresenius is a health care group with international operations, providing products and services for dialysis, hospital and outpatient medical care. In 2006, group sales were approx. € 10.8 billion. On September 30, 2007 the Fresenius Group had 110,379 employees worldwide.
TRION Pharma is a biopharmaceutical company that develops and produces trifunctional antibodies based on a globally patented technology platform together with Fresenius Biotech in Munich. For further information please visit www.trionpharma.de.
Glossary
Epithelial tumors: tumors that result from degenerated cells of epithelial origin.
This release contains forward-looking statements that are subject to various risks and uncertainties. Actual results could differ materially from those described in these forward-looking statements due to certain factors, including changes in business, economic and competitive conditions, regulatory reforms, foreign exchange rate fluctuations, uncertainties in litigation or investigative proceedings, and the availability of financing. Fresenius does not undertake any responsibility to update the forward-looking statements in this release.
Board of Management: Dr. Ulf M. Schneider (President and CEO), Rainer Baule, Andreas Gaddum, Dr. Jürgen Götz, Dr. Ben Lipps, Stephan Sturm
Supervisory Board: Dr. Gerd Krick (Chairman)
Registered Office: Bad Homburg, Germany/Commercial Register No. HRB 10660
Fresenius AG today provided details regarding its contemplated bond issue announced in October 2005. The Company intends to issue € 1.0 billion in Senior Notes (the "Senior Notes") through its wholly-owned subsidiary Fresenius Finance B.V. (the "Issuer"), subject to market conditions. The net proceeds from the Senior Notes offering (the "Senior Notes Offering"), along with the proceeds from our recent capital increase, finance the acquisition of HELIOS Kliniken GmbH and the proposed repurchase of the Issuer's outstanding € 300 million 7.750 % Series A Senior Notes due 2009, callable in 2006, by way of a cash tender (the "Tender Offer") and will be used for general corporate purposes.
Fresenius AG expects to complete the Senior Notes Offering before the end of January 2006.
The proposed Senior Notes Offering will not be registered under the Securities Act of 1933, but will be offered in the United States pursuant to an exemption from registration under Rule 144A, as well as outside the United States under Regulation S.
Credit Suisse First Boston (Europe) Limited and Morgan Stanley & Co. International Limited have been mandated as Joint Book-Running Lead Managers and Dresdner Bank AG London Branch as Joint Lead Manager of the proposed Senior Notes Offering.
The Tender Offer is subject to the terms described in the tender offer memorandum dated January 3, 2006.
Fresenius is a health care group with international operations, providing products and services for dialysis, hospital and the ambulatory medical care of patients. In 2004, sales were € 7.27 billion. On December 31, 2004 the Fresenius Group had 68,494 employees worldwide.
This announcement is for distribution only to persons who (i) have professional experience in matters relating to investments falling within Article 19(5) of the Financial Services and Markets Act 2000 (Financial Promotion) Order 2005 (as amended, the "Financial Promotion Order"), (ii) are persons falling within Article 43 of the Financial Promotion Order (iii) are persons falling within Article 49(2)(a) to (d) ("high net worth companies, unincorporated associations etc") of the Financial Promotion Order, (iv) are outside the United Kingdom, or (v) are persons to whom this announcement can otherwise be lawfully communicated or caused to be communicated (all such persons together being referred to as "relevant persons"). This announcement is directed only at relevant persons and must not be acted on or relied on by persons who are not relevant persons. Any investment or investment activity to which this announcement relates is available only to relevant persons and will be engaged in only with relevant persons.
This announcement is not an offer for sale of securities in the United States. Securities may not be sold in the United States absent registration or an exemption from registration under the U.S. Securities Act of 1933, as amended (re "Securities Act"). The Senior Notes referred to herein have not been and will not be registered under the Securities Act and Fresenius does not intend to register any portion of the offering in the United States or to conduct a public offering of securities in the United States. Copies of this announcement are not being made and may not be distributed or sent into the United States, Canada, Japan or Australia. It may be unlawful to distribute this announcement in certain other jurisdictions. The information in this announcement does not constitute an offer of securities for sale in Canada, Japan or Australia.
This announcement or any related documents are not being distributed in the context of a public offer in France within the meaning of Article L. 411-1 of the French Monetary and Financial Code (Code monétaire et financier), and thus neither this announcement nor any prospectus has been or will be submitted to the Autorité des Marchés Financiers for approval in France and accordingly may not and will not be distributed to the public in France. The offer of the Senior Notes is not being made and will not be made to the public in France except to (i) qualified investors (investisseurs qualifiés) and/or a restricted group of investors (cercle restreint d'investisseurs), in each case, acting for their own account, all as defined in, and in accordance with, Articles L. 411-1, L. 411-2, D. 411-1 and D. 411-2 of the French Monetary and Financial Code and/or (ii) persons providing portfolio management investment services acting for third parties.
This announcement does not constitute an offer to sell notes or a solicitation to buy notes in Germany. There will be no public offering of notes in Germany. Any offering of notes can only be made in accordance with the German Securities Prospectus Act (Wertpapierprospektgesetz, WpPG). No selling document pursuant to the German Securities Prospectus Act has been or will be published in relation to the Senior Notes. Therefore, this announcement and any other offering material in relation to the Senior Notes is directed only at persons who are "qualified investors" within the meaning of sec. 2 No. 6 of the German Securities Prospectus Act.
This announcement is an advertisement and is not a prospectus for the purposes of EU Directive 2003/71/EC (the "Directive"). This announcement and the offering of Senior Notes when made are only addressed to and directed at persons in member states of the European Economic Area who are "qualified investors" within the meaning of Article 2(1)(e) of the Directive.
Fresenius Medical Care ("the Company") (Frankfurt Stock Exchange: FME - ISIN: DE0005785802, FME3 - ISIN: DE0005785836) (NYSE: FMS, FMS-p), the world's largest provider of Dialysis Products and Services, today announced that the preference share conversion offer will start on January 6, 2006. All preference shareholders, including holders of preference shares represented by American Depositary Shares ("ADSs") will have the opportunity to convert their preference shares into ordinary shares on a 1:1 basis accompanied by payment of a conversion premium of €9.75 per preference share to the Company in a period of four weeks ending February 3, 2006, subject to extension. The share conversion and transformation of the legal form of the Company into a KGaA are expected to be completed during February 2006.
The conversion offer is being conducted through two offers – a German offer and a U.S. offer open to all preference shareholders resident in the U.S. and all ADS holders. All terms and conditions of the conversion offers are detailed in the German prospectus, which is also available for download from our webpage (www.fmc-ag.com), and the U.S. prospectus, which will be mailed to U.S. resident shareholders and ADS holders. Additional copies of the German prospectus may be obtained from the Company or from Deutsche Bank AG; additional copies of the U.S. prospectus may be obtained from D.F. King & Co., Inc., the Information Agent for the U.S. offer.
# # #
Fresenius Medical Care AG is the world's largest, integrated provider of products and services for individuals undergoing dialysis because of chronic kidney failure, a condition that affects more than 1,300,000 individuals worldwide. Through its network of approximately 1,670 dialysis clinics in North America, Europe, Latin America, Asia-Pacific and Africa, Fresenius Medical Care provides dialysis treatment to approximately 130,400 patients around the globe. Fresenius Medical Care is also the world's leading provider of dialysis products such as hemodialysis machines, dialyzers and related disposable products.
For more information about Fresenius Medical Care visit the Company's website at www.fmc-ag.com.
The conversion offer may be made in the United States only by prospectus. The conversion offer has not yet commenced. When the conversion offer commences, each United States resident preference shareholder of Fresenius Medical Care AG should read the prospectus when it becomes available because it will contain important information about the conversion offer. Fresenius Medical Care preference shareholders can obtain the U.S. prospectus and other documents that are filed with the United States Securities and Exchange Commission's web site at www.sec.gov. Preference shareholders may also obtain copies of the prospectus and other documents filed with the Securities and Exchange Commission for free by contacting Fresenius Medical Care when the documents become available.



