Fresenius Medical Care AG & Co. KGaA ("the Company"), the world's largest provider of Dialysis Products and Services, today announced that the share split with capital increase from the Company's funds approved by the Ordinary General Meeting on May 15, 2007, will become effective on June 18, 2007. On the same day, the shares will be traded "ex split" and the shareholders' deposits will be adapted to the new number of shares. Every holder of an ordinary share now holds three ordinary shares and every holder of a preference share holds three preference shares. As a result of the share split, the price level will be reduced arithmetically without affecting the overall value for shareholders.
The Fresenius Medical Care shares will continue to trade under ISIN DE0005785802 (ordinary share) and ISIN DE0005785836 (preference share).
The subscribed capital of Fresenius Medical Care AG & Co. KGaA now amounts to €295,422,342.00, divided into 291,701,520 ordinary shares and 3,720,822 preference shares.
For American Depositary Share (ADS) Investors:
Fresenius Medical Care shares are traded on the New York Stock Exchange (NYSE) in the form of ADSs under the ISIN US3580291066 (ordinary share) and ISIN US3580292056 (preference share). Before the share split 3 ADSs represented 1 underlying Share. Upon effectiveness of the share split, the ratio between the ordinary and preference ADS and the underlying ordinary and preference shares is now 1:1, meaning that one Fresenius Medical Care ordinary or preference ADSs is the equivalent of one Fresenius Medical Care ordinary or preference share.
For more information about Fresenius Medical Care visit the Company's website at www.fmc-ag.com.
# # #
Fresenius Medical Care is the world's largest integrated provider of products and services for individuals undergoing dialysis because of chronic kidney failure, a condition that affects more than 1,500,000 individuals worldwide. Through its network of 2,194 dialysis clinics in North America, Europe, Latin America, Asia-Pacific and Africa, Fresenius Medical Care provides dialysis treatment to 169,216 patients around the globe. Fresenius Medical Care is also the world's leading provider of dialysis products such as hemodialysis machines, dialyzers and related disposable products. Fresenius Medical Care is listed on the Frankfurt Stock Exchange (FME, FME3) and the New York Stock Exchange (FMS, FMS/P).
Fresenius Medical Care AG & Co. KGaA ("the Company") (Frankfurt Stock Exchange: FME, FME3) (NYSE: FMS, FMS-p), the world's largest provider of Dialysis Products and Services, today announced that it intends to sell approximately US$ 500 million senior unsecured notes. The notes will be offered mainly to US institutional investors. Proceeds from the offering will be used to reduce indebtedness under the Company's senior secured bank credit facility and other, short-term debt, and for general corporate purposes.
The proposed offering will not be registered under the Securities Act of 1933, but will be offered in the United States pursuant to an exemption from registration under Rule 144A as well as outside the United States under Regulation S. The Company expects completion of the offering at the beginning of July 2007.
# # #
Fresenius Medical Care is the world's largest integrated provider of products and services for individuals undergoing dialysis because of chronic kidney failure, a condition that affects more than 1,500,000 individuals worldwide. Through its network of 2,194 dialysis clinics in North America, Europe, Latin America, Asia-Pacific and Africa, Fresenius Medical Care provides dialysis treatment to 169,216 patients around the globe. Fresenius Medical Care is also the world's leading provider of dialysis products such as hemodialysis machines, dialyzers and related disposable products. Fresenius Medical Care is listed on the Frankfurt Stock Exchange (FME, FME3) and the New York Stock Exchange (FMS, FMS/P).
For more information about Fresenius Medical Care visit the Company's website at www.fmc-ag.com.
Most dialysis patients have a significantly better chance of survival if they are treated with high-flux dialyzers rather than low-flux dialyzers. This is the conclusion of a new international study conducted under the direction of the Italian renal specialist Prof. Francesco Locatelli from the Alessandro Manzoni Hospital in Lecco.
The results of the study were presented last weekend during the European Dialysis and Transplantation Association/European Renal Association (EDTA/ERA) Congress in Barcelona. The study showed that dialysis patients with a low albumin concentration in their blood that were treated with high-flux dialyzers had a 37% lower mortality risk during the three to seven-and-a-half years of the study than those that were treated with low-flux dialyzers. Depending on the country, 56% to 85% of dialysis patients have a low albumin concentration in their blood (four grams per deciliter or less).
The study was conducted over seven-and-a-half years in nine European countries. The 738 patients involved were treated three times a week. Half of the patients received treatment with high-flux dialyzers – predominantly dialyzers from Fresenius Medical Care. The other half received therapy with low-flux dialyzers.
This is the first time a prospective randomized clinical study scientifically proves that treatment with high-flux dialyzers reduces the mortality risk of patients with severe chronic kidney disease. Indications of a lower mortality risk first appeared in the mid-90s.
Specialists attribute the increased survival rates from high-flux dialyzers to a more efficient filtering of larger uremic toxins from the blood. High-flux membranes have a greater water permeability and pores that are two-and-a-half times larger than low-flux membranes. The filtering capabilities of high-flux membranes are closer to the natural kidney function and allow the body to remove large amounts of liquids and toxic uremic substances in a short period of time. High-flux dialyzers can also help maintain any remaining kidney function for a longer period of time.
High-flux dialyzers have the most technically advanced membranes. Their use is increasing worldwide and, in many countries, more than 60% of the patients are treated with high-flux dialyzers. "The positive results of the new study validate our efforts to offer innovative dialysis products such as our high-flux dialyzers with Helixone membranes so that dialysis patients can look toward the future with more confidence. We expect demand for high-flux dialyzers to continue to increase. And we are proud that the majority of the patients in the study's high-flux-group were treated with our dialyzers," said Dr. Emanuele Gatti, Fresenius Medical Care Chief Executive Officer for Europe, Latin America, Middle East and Africa.
# # #
Helixone is a registered trademark of Fresenius Medical Care.
Fresenius Medical Care is the world's largest integrated provider of products and services for individuals undergoing dialysis because of chronic kidney failure, a condition that affects more than 1,500,000 individuals worldwide. Through its network of 2,194 dialysis clinics in North America, Europe, Latin America, Asia-Pacific and Africa, Fresenius Medical Care provides dialysis treatment to 169,216 patients around the globe. Fresenius Medical Care is also the world's leading provider of dialysis products such as hemodialysis machines, dialyzers and related disposable products. Fresenius Medical Care is listed on the Frankfurt Stock Exchange (FME, FME3) and the New York Stock Exchange (FMS, FMS/P).
For more information about Fresenius Medical Care visit the Company's website at www.fmc-ag.com.
Fresenius Medical Care AG & Co. KGaA ("the Company"), the world's largest provider of Dialysis Products and Services, today announced the pricing of Senior Notes due 2017 in the amount of US$ 500 million. The coupon will be 6 7/8%. Proceeds will be used to reduce indebtedness under the Company's senior secured bank credit facility and other, short-term debt.
The Senior Notes will be issued by FMC Finance III S.A., a wholly-owned subsidiary of the Company, and will be guaranteed on a senior basis jointly and severally by the Company, Fresenius Medical Care Holdings, Inc. and Fresenius Medical Care Deutschland GmbH.
Lawrence A. Rosen, Chief Financial Officer of Fresenius Medical Care, commented: "We are pleased to have successfully completed the company's first senior unsecured bond offering. Investors have clearly recognized our sustainable financial strength and are confident in the future of the industry and Fresenius Medical Care."
The notes were not registered under the Securities Act of 1933, but were offered in the United States pursuant to an exemption of registration under Rule 144 A, as well as outside the United States under Regulation S. The notes may not be offered or sold in the United States without registration or an applicable exemption from the registration requirements.
# # #
Fresenius Medical Care is the world's largest integrated provider of products and services for individuals undergoing dialysis because of chronic kidney failure, a condition that affects more than 1,500,000 individuals worldwide. Through its network of 2,194 dialysis clinics in North America, Europe, Latin America, Asia-Pacific and Africa, Fresenius Medical Care provides dialysis treatment to 169,216 patients around the globe. Fresenius Medical Care is also the world's leading provider of dialysis products such as hemodialysis machines, dialyzers and related disposable products. Fresenius Medical Care is listed on the Frankfurt Stock Exchange (FME, FME3) and the New York Stock Exchange (FMS, FMS/P).
For more information about Fresenius Medical Care visit the Company's website at www.fmc-ag.com.
Fresenius today announced that the conversion of Fresenius AG into a European Company (Societas Europaea) took effect on July 13, 2007 with the entry of Fresenius SE in the commercial register of the Bad Homburg municipal court. The Company's Supervisory Board will continue to have twelve members, divided equally between employee and shareholder representatives.
For the first time, employees from other European countries outside of Germany will have Board representation with one member representing Austrian employees and another representing Italian employees. The six shareholder representatives for the new Supervisory Board were already appointed as part of the December 4, 2006 Extraordinary General Meeting that had approved the statutes of Fresenius SE. During that Meeting, a vast majority of 99.99 percent of the ordinary share capital represented had approved the conversion.
An SE is a public limitedliability company under European law. The SE particularly facilitates an open and international corporate culture while reflecting Fresenius' global orientation. With the exception of the composition of the Supervisory Board, the conversion will have no effect on the Company's management structure.
# # #
Fresenius is a health care group with international operations, providing products and services for dialysis, hospital and outpatient medical care. In 2006 group sales were about € 10.8 billion. On December 31, 2006 the Fresenius Group had 107,348 employees worldwide.
Fresenius today announced that further secondary endpoint data from a phase II/III study with removab® (catumaxomab) in patients with malignant ascites confirm clear benefits for patients treated with the antibody. Trial data show that removab significantly increases time to tumor progression and has a positive influence on overall survival time. Moreover, a prolonged interval between punctures was seen in the removab group compared to the control group and this effect was also observed beyond the end of study.
The results of the randomized study include treatment data from 258 patients with malignant ascites caused by various cancers. Most patients had late-stage disease with a median life expectancy of two to three months. The primary endpoint of the study had already demonstrated that patients receiving removab had a four-fold increased puncture-free survival over a therapy with puncture alone (median 46 vs. 11 days, p<0.0001). The median time to the first therapeutic puncture, a key secondary endpoint, improved to 77 days in the removab group versus 13 days in the control group (p<0.0001). In contrast to the primary endpoint, patients who died before the next puncture were not included in this metric.
There was a clear difference between the two study arms with regard to the time to progression (TTP) of the underlying cancer. The median TTP (secondary endpoint) for the 170 patients treated with removab was 111 days, compared to 35 days for the 88 patients of the control group (p<0.0001). In the subgroup of patients with ascites from ovarian cancer, removab-treated TTP was also 111 days, compared to patients in the control group with 35 days (p=0.0002). In addition, patients with other primary cancers receiving removab also showed significant improvement in TTP, with a median of 110 days versus 34 days with puncture-therapy alone (p<0.0001).
A positive trend was also observed for overall survival. Median overall survival in the 170 patients in the removab group was 72 days, compared to 68 days of the 88 patients in the control group (p=0.0846). In a prospectively planned evaluation of 131 patients treated per protocol, a median survival advantage of 18 days was shown (removab: 86 days vs. control group: 68 days, p=0.0085). removab treatment also showed a positive influence on the overall survival of ovarian cancer patients with a median survival of 110 days over 81 days for patients receiving puncture-therapy alone (p=0.1543). In patients with gastric cancer (the largest subgroup among patients with cancers other than ovarian cancer) the median survival advantage was 27 days (71 vs. 44 days, p=0.0313).
After the primary end point of the study (puncture-free survival) was reached, the data showed that puncture intervals in patients treated with removab continued to be longer than those of the patients in the control group. The intervals between the first and second puncture were 26 and 24 days in the ovarian and non-ovarian cancer group treated with removab vs. 13 and 16 days in the control group.
Secondary study endpoints and other data collected from patients after reaching the primary endpoint confirm the benefit of removab treatment for this patient group at a late stage of their disease. Collectively, the study results further indicate the efficacy of removab in the treatment of various primary cancers. "The results of the phase II/III study show clear benefits for patients being treated with removab", says Dr. Bernhard Ehmer, President Fresenius Biotech. "The results also suggest a direct antitumor effect of the trifunctional antibody. Primary and secondary endpoints as well as post study data are consistent, and demonstrate a pronounced positive trend. They all point in the right direction and this gives us confidence in our ongoing development program for ovarian and gastric cancer."
Fresenius Biotech confirms that the submission for marketing authorization with EMEA is expected in late 2007.
# # #
About the phase II/III study with removab
A total of 258 patients with end-stage cancer and recurrent symptomatic malignant ascites, who either no longer responded to or could no longer be treated with chemotherapy, were enrolled in this study. 129 of the patients had ovarian cancer and 129 participants had other forms of epithelial tumors (gastric 51 %, breast 10 %, pancreatic 7 %, colorectal 6 %, others 26 %). Participants in the study were randomized in a 2:1 ratio, with 170 patients randomized to treatment with removab and 88 patients to puncture therapy alone. After reaching the study endpoint 51% of patients in the control arm were also given removab (crossover). removab was administered intraperitoneally at ascending doses through infusions, following paracentesis on days 0, 3, 7, and 10. 131 patients received all four infusions of 10, 20, 50, and 150 µg.
Mode of action of trifunctional antibody removab (catumaxomab)
The therapeutic objective of trifunctional antibodies is to generate a stronger immune reaction against tumor cells. removab has two different antigen binding sites: While one arm of the antibody recognizes and binds to T-cells, the other arm binds EpCAM (epithelial cell adhesion molecule) that is overexpressed in many types of epithelial cancers. Immune effector cells with Fc receptors (macrophages, monocytes, dendritic cells and natural killer cells) can also bind the Fc region of intact trifunctional antibodies. This simultaneous binding subsequently results in the costimulation and activation of T-cells and accessory cells, enabling the generation of a strong immune response against tumor cells. Preclinical data also suggest a potential long-lasting effect to prevent cancer recurrence. Apart from removab two other trifunctional antibodies targeting other cancer antigens are currently undergoing clinical development.
Trifunctional Antibodies
Trifunctional antibodies are proteins that activate different cell types of the immune system simultaneously and target tumor cells specifically. Trifunctional antibodies therefore are very effective in destroying cancer cells and show a therapeutic effect even at very low doses. They are being developed by TRION Pharma GmbH.
# # #
Fresenius Biotech is a company within the Fresenius health care group and is focused on the development and marketing of biopharmaceuticals in the fields of oncology, immunology and regenerative medicine. For further information please visit www.fresenius-biotech.com.
Fresenius is a health care group with international operations, providing products and services for dialysis, hospital and outpatient medical care. In 2006, group sales were about 10.8 billion. On March 31, 2007 the Fresenius Group had 107.348 employees worldwide.
Trion Pharma is a biopharmaceutical company that develops and produces trifunctional antibodies based on a globally patented technology platform together with Fresenius Biotech in Munich. For further information please visit www.trionpharma.de.
# # #
Glossary
Puncture-free survival period: Period between the last infusion (control group: day of the puncture) and the first subsequent necessary puncture or death, which ever occurs first.
TTP (Time to Progression): Time to progression is the length of time between treatment and further growth of the primary tumor or metastases.
Summary Second Quarter 2007
- Net revenue : $ 2,404 million, + 11%
- Operating income (EBIT): $ 391 million, + 5%
- Net income: $ 179 million, + 38%
- Earnings per share: $ 0.60, + 37%
Summary First Half 2007
- Net revenue : $ 4,725 million, + 21%
- Operating income (EBIT): $ 756 million, + 23%
- Net income: $ 339 million, + 38%
- Earnings per share: $ 1.15, + 37%
Fresenius Medical Care AG & Co. KGaA ("the Company"), the world's largest provider of Dialysis Products and Services, today announced its results for the second quarter and first half 2007.
Second Quarter 2007:
Revenue
Net revenue for the second quarter 2007 increased by 11% to $2,404 million (9% at constant currency) compared to the second quarter 2006. Organic revenue growth worldwide was 8%. Dialysis Services revenue grew by 9% to $1,796 million (8% at constant currency) in the second quarter of 2007. Dialysis Product revenue increased by 18% to $608 million (13% at constant currency) in the same period.
North America revenue increased by 6% to $1,660 million. Dialysis Services revenue grew by 5% to $1,499 million. Excluding effects of the divestiture of the perfusion business, Dialysis Service revenue increased by 6%. Average revenue per treatment for the U.S. clinics increased by 3% to $327 in the second quarter 2007 compared to $317 for the same quarter in 2006. Dialysis Product revenue increased by 21% to $161 million led by strong sales of our 2008K hemodialysis machines and the phosphate binding drug PhosLo.
International revenue was $744 million, an increase of 23% (15% at constant currency) compared to the second quarter of 2006. Dialysis Services revenue reached $296 million, an increase of 32% (24% at constant currency). Dialysis Product revenue rose by 17% to $448 million (10% at constant currency), led by strong sales of hemodialysis machines, peritoneal dialysis products and dialyzers.
Earnings
Operating income (EBIT) increased by 5% to $391 million compared to $372 million in the second quarter 2006. Operating income for the second quarter 2006 includes costs of $4 million related to costs of restructuring and the transformation of the Company's legal form, and a gain of $39 million from the divestiture of dialysis clinics in conjunction with the acquisition of Renal Care Group. Excluding these costs and the gain from the divestiture, operating income for the second quarter 2007 increased by 16%, resulting in an operating margin of 16.3%. For the second quarter 2006 the operating margin was 15.5%.
In North America, compared with the second quarter 2006, the operating margin excluding the effects of one-time items increased by 140 basis points to 17.2% due to revenue rate improvements, the new PhosLo business and higher product sales which more than offset higher personnel expenses. In the International segment, the operating margin decreased by 50 basis points to 17.5% mainly due to higher growth in the dialysis care business.
Net interest expense for the second quarter 2007 was $92 million compared to $100 million in the same quarter of 2006. This positive development was mainly attributable to a lower debt level in combination with lower average interest rates.
Income tax expense was $113 million for the second quarter of 2007 compared to $137 million in the second quarter of 2006, reflecting effective tax rates of 38.0% and 50.6%, respectively. In the second quarter 2006, the tax rate had been impacted by a tax expense related to the gain on the divestiture of dialysis clinics in the U.S. Excluding this impact, the tax rate was at 40.2%.
Net income for the second quarter 2007 was $179 million, an increase of 38%. Net income increased by 30% when compared to the second quarter 2006 excluding the effects of one-time items in 2006.
Earnings per share (EPS) for the second quarter of 2007 rose by 37% to $0.60 per ordinary share compared to $0.44 for the second quarter of 2006. Earnings per ordinary American Depository Share (ADS) are the same as one ADS now represents one share as a result of the change in ratio under the Company's ordinary shares and preference shares. The weighted average number of shares outstanding for the second quarter of 2007 was approximately 295.4 million shares compared to 293.9 million shares for the second quarter of 2006. The increase in shares outstanding results from stock option exercises in 2006 and in the first half 2007.
Cash Flow
In the second quarter of 2007, the Company generated $225 million in cash from operations, representing 9% of revenue. The strong cash flow generation was primarily supported by earnings.
A total of $132 million was spent for capital expenditures, net of disposals. Free Cash Flow before acquisitions was $93 million compared to $145 million in the second quarter of 2006 excluding the effects of the acquisition of RCG. A total of $24 million in cash was used for acquisitions. Free Cash Flow after acquisitions was $69 million compared to $121 million last year, excluding the acquisition of Renal Care Group.
First Half 2007:
The operations of Renal Care Group (RCG) are included in the Company's consolidated statements of income and cash flows from April 1, 2006, therefore, the current first half year results are not directly comparable with the results of the first six months for 2006.
Revenue and Earnings
Net revenue was $4,725 million, up 21% from the first half of 2006. At constant currency, net revenue rose by 19%. Organic growth was 8% in the first six months of 2007.
Operating income (EBIT) increased by 23% to $756 million compared to $616 million in the first half of 2006. Operating income for the first half of 2006 includes costs of $4 million as a result of restructuring and the transformation of the Company's legal form, and a gain from the clinic divestitures of $39 million.
Excluding these items, operating income for the first half of 2007 increased by 30%. This performance resulted in an operating margin of 16.0% compared to 14.8% for the first half of 2006.
Net interest expense for the first six months of 2007 was $187 million compared to $156 million in the same period of 2006. The increase was the result of additional interest expense partially offset by the write-off in 2006 of deferred financing costs related to the 2003 senior credit facility of $15 million, both in conjunction with the financing of the RCG acquisition.
Income tax expense was $216 million in the first half of 2007 compared to $209 million in the same period in 2006, reflecting tax rates of 38.0% and 45.4%, respectively. The tax rate in the first half of 2006 was impacted by a tax expense related to the gain on the divestiture of dialysis clinics in the U.S. Excluding this impact, the effective tax rate in the first half of 2006 was at 39.2%.
For the first half of 2007, net income was $339 million, up 38% from the first half of 2006. Net income for the first half of 2007 increased by 29% compared to the first half of 2006 excluding the effects of one-time items in 2006.
For the first half of 2007, earnings per ordinary share rose by 37% to $1.15. The weighted average number of shares outstanding during the first half of 2007 was approximately 295.3 million.
Cash flow
Cash from operations during the first six months of 2007 was $508 million compared to $312 million for the same period in 2006 on a reported basis. Excluding the effects of one-time items, cash from operations was $402 million in the first half of 2006. The increase compared to prior year was mainly due to increased earnings.
A total of $240 million was used for capital expenditures, net of disposals. Free Cash Flow before acquisitions for the first six months of 2007 was $268 million compared to $152 million in same period in 2006. The underlying Free Cash Flow before acquisitions and the effects of one-time items for the first half of 2006 was $242 million. A total of $114 million in cash was used for acquisitions.
Please refer to the attachments for a complete overview on the second quarter and first half 2007.
Patients – Clinics – Treatments
As of June 30, 2007, Fresenius Medical Care treated 171,687 patients worldwide, which represents a 6% increase in patients compared to last year. North America provided dialysis treatments for 120,270 patients, an increase of 2%. Including 32 clinics managed by Fresenius Medical Care North America, the number of patients in North America was 122,199. The International segment served 51,417 patients, an increase of 17% over last year.
As of June 30, 2007, the Company operated a total of 2,209 clinics worldwide. This is comprised of 1,581 clinics in North America, an increase of 3%, and 628 clinics in the International segment, an increase of 17%.
Fresenius Medical Care delivered approximately 13.0 million dialysis treatments worldwide during the first six months of 2007. This represents an increase of 16% year over year. North America accounted for 9.08 million treatments, an increase of 16%, and the International segment delivered 3.92 million treatments, an increase of 17% over last year.
Employees
As of June 30, 2007, Fresenius Medical Care had 60,031 employees (full-time equivalents) worldwide compared to 56,803 employees at the end of 2006. The increase of 3,228 employees is primarily due to acquisitions in Asia and continued organic growth in the U.S.
Debt/EBITDA Ratio
The ratio of debt to Earnings before Interest, Taxes and Amortization (EBITDA) decreased from 3.60 at the end of the second quarter of 2006 to 3.03 at the end of the second quarter 2007. At the end of 2006, the debt/EBITDA ratio was 3.23.
Rating
In the second quarter 2007, Standard & Poor's Ratings Services raised its rating on the Company's senior secured debt to 'BBB-' from 'BB+'. Standard & Poor's also upgraded the outlook for the Company's corporate rating from "negative" to "stable".
Moody's upgraded the outlook for Fresenius Medical Care to ‘positive' from ‘stable'.
Issuance of 10 Year Senior Notes
At the beginning of the third quarter 2007, Fresenius Medical Care issued Senior Notes due 2017 in the amount of $500 million. The coupon is 6 7/8%. Proceeds were used to reduce indebtedness under the Company's senior secured bank credit facility and other, short-term debt. The Senior Notes were issued by FMC Finance III S.A., a wholly-owned subsidiary of the Company, and are guaranteed on a senior basis jointly and severally by the Company, Fresenius Medical Care Holdings, Inc. and Fresenius Medical Care Deutschland GmbH.
Acquisition of a Production Plant in China
On July 17, 2007 Fresenius Medical Care acquired a production plant in Jiangsu, China from Bioteque Corp., Taipei, Taiwan. This plant currently produces bloodlines and other non-reusable products for the Chinese market and offers excellent additional opportunities to produce liquid and other non-reusable products for the Chinese market and other countries in the region. In addition, the Company entered into three exclusive distribution contracts for marketing and distribution of Bioteque's bloodline and needle products in Taiwan, Korea and Japan.
Divestiture of Perfusion Business in the U.S.
Fresenius Medical Care sold the perfusion business unit of Fresenius Medical Care Extracorporeal Alliance ("FMCEA") to Specialty Care Services Group, Inc. during the second quarter 2007. In 2006, FMCEA's perfusion business contributed revenue of approximately $110 million. The Company deconsolidated the U.S. perfusion business effective May 9, 2007.
Share Split of 1:3
On June 18, 2007, the previously announced share split for both classes of shares (ordinary and preference) in the ratio of 1:3 became effective. In connection with the share split, the ratio between the ordinary and preference ADS and the underlying ordinary and preference shares was adjusted to 1:1, meaning that one Fresenius Medical Care ordinary or preference ADS is now the equivalent of one Fresenius Medical Care ordinary or preference share.
Outlook for 2007 Upgraded
Based on the strong operational performance in the first half of 2007, the Company raises its outlook for the full year 2007 and now expects to achieve revenue of more than $9.5 billion. This represents an increase of at least 12%. Previously, the Company expected revenue of approximately $9.4 billion.
Net income is now projected to be in the range of $685 million to $705 million in 2007. This represents an increase of between 19% and 23% on an adjusted basis as compared to 2006 after one-time effects. On a reported basis, this translates into an increase in net income of between 28% and 31%. Previously, the Company expected net income in the range of $675 million to $695 million.
In addition, the Company still expects spending on capital expenditures and acquisitions to be approximately $650 million in 2007. The debt/EBITDA ratio is projected to be below 3.0 by the end of 2007.
Ben Lipps, Chief Executive Officer of Fresenius Medical Care, commented: "We are pleased to report excellent financial results for the second quarter and six months ending June 30, 2007. Contributing to the financial results, we have achieved an organic growth rate of 8%. In addition we readjusted our service portfolio focusing on profitability and expanded our product base in Asia-Pacific. We continue to see many growth opportunities and upgraded our guidance which reflects our confidence in the further profitable growth of our company particularly in the international region. We remain focused on providing quality care for our patients, working on all fronts to ensure that they achieve the best possible clinical outcomes to maximize their overall health and well being."
# # #
Fresenius Medical Care is the world's largest integrated provider of products and services for individuals undergoing dialysis because of chronic kidney failure, a condition that affects more than 1,500,000 individuals worldwide. Through its network of 2,209 dialysis clinics in North America, Europe, Latin America, Asia-Pacific and Africa, Fresenius Medical Care provides dialysis treatment to 171,687 patients around the globe. Fresenius Medical Care is also the world's leading provider of dialysis products such as hemodialysis machines, dialyzers and related disposable products. Fresenius Medical Care is listed on the Frankfurt Stock Exchange (FME, FME3) and the New York Stock Exchange (FMS, FMS/P).
For more information about Fresenius Medical Care visit the Company's website at www.fmc-ag.com.
Fresenius Medical Care
Statement of Earnings see PDF-file
- Sales: € 5.59 billion, + 10 % at actual rates, + 15 % in constant currency
- EBIT: € 780 million, + 15 % at actual rates, + 20 % in constant currency
- Net income: € 195 million, + 39 % at actual rates, + 44 % in constant currency
- Excellent sales and earnings growth and further margin improvements in all business segments
Earnings outlook for 2007 raised
Based on the Group's strong financial results in the first half, Fresenius raises its earnings outlook for 2007. Net income is now expected to increase by ~25 % in constant currency. Previously, the Company expected net income to increase by 20 to 25 %. Group sales are expected to grow by 8 to 10 % in constant currency.
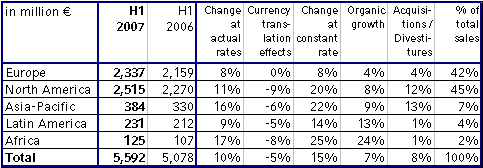
Sales – excellent organic growth
Group sales increased by 10 % to € 5,592 million (H1 2006: € 5,078 million). Organic sales growth was 7 %. Acquisitions contributed 10 %, in particular Renal Care Group, which was consolidated as from the second quarter of 2006. Divestitures reduced sales by 2 %. Currency translation effects had a negative impact of 5 %. This was mainly attributable to the average dollar rate depreciating 8 % against the euro in the first half of 2007 compared to previous year's period.
In North America sales grew significantly due to the Renal Care Group consolidation and an excellent organic growth rate of 8 %. In Europe sales increased by 8 % in constant currency, with organic growth of 4 %. Strong growth rates were achieved in the emerging markets with organic growth of 9 % in Asia-Pacific, 13 % in Latin America and 24 % in Africa.

Strong earnings growth
EBITDA increased by 18 % in constant currency and by 13 % at actual rates to € 977 million (H1 2006: € 867 million). Group operating income (EBIT) increased by 20 % in constant currency and by 15 % at actual rates to € 780 million (H1 2006: € 681 million; adjusted € 652 million including a gain from the divestiture of US dialysis clinics and one-time expenses related to the Renal Care Group acquisition). The strong earnings growth was driven by the successful operating results in all business segments. The Group's EBIT margin improved to 13.9 % (H1 2006: 13.4 %, adjusted margin: 12.8 %).
Group net interest was € -185 million (H1 2006: € -194 million, including one-time expenses of € 30 million for the early refinancing of Group debt).
The tax rate improved to 36.0 % (H1 2006: 42.3%; 37.4 % adjusted for the tax expense related to the divestiture of US dialysis clinics).
Minority interest increased to € 186 million (H1 2006: € 141 million), of which 93 % was attributable to the minority interest in Fresenius Medical Care.
Group net income also grew strongly by 44 % in constant currency and by 39 % at actual rates to € 195 million (H1 2006: € 140 million, including one-time expenses of € 16 million).
Earnings per ordinary share were € 1.26 and earnings per preference share were € 1.27 (H1 2006, adjusted for the February 2007 share split: ordinary share € 0.91, preference share € 0.92). This represents an increase of 38 % for both share classes.
High level of investment
Fresenius Group spent € 304 million for property, plant and equipment and intangible assets (H1 2006: € 225 million). Acquisition spending was € 221 million (H1 2006: € 3,408 million).
Strong cash flow
Operating cash flow increased by 48 % to € 553 million (H1 2006: € 373 million), mainly driven by the strong earnings increase. Cash flow before acquisitions and dividends increased by 60 % to € 256 million (H1 2006: € 160 million). Free cash flow after acquisitions (€ 162 million) and dividends (€ 188 million) was € -94 million (H1 2006: € -2,997 million).
Solid balance sheet structure
Total assets increased by 3 % in constant currency and by 2 % at actual rates to € 15,343 million (December 31, 2006: € 15,024 million). Current assets increased by 4 % to € 4,275 million (December 31, 2006: € 4,106 million). Non-current assets were € 11,068 million (December 31, 2006: € 10,918 million).
Shareholders' equity including minority interest grew by 3 % to € 5,895 million (December 31, 2006: € 5,728 million). The equity ratio (including minority interest) was 38.4 % (December 31, 2006: 38.1 %).
The Group's debt was € 5,909 million (December 31, 2006: € 5,872 million). The net debt/EBITDA ratio stood at 2.9 on June 30, 2007 (December 31, 2006: 3.0).
Employees
As of June 30, 2007, the Group had 108,860 employees (December 31, 2006: 104,872), an increase of 4 %.
Fresenius Biotech
Fresenius Biotech develops innovative therapies with trifunctional antibodies for the treatment of cancer as well as cell therapies for the treatment of the immune system. In the field of polyclonal antibodies, Fresenius Biotech has successfully marketed ATG-Fresenius S for many years. ATG-Fresenius S is an immunosuppressive agent used to prevent and treat graft rejection following organ transplantation.
In July 2007, Fresenius Biotech announced further secondary endpoint data from a phase II/III study with removab® (catumaxomab) in patients with malignant ascites. The data confirmed clear benefits for patients treated with the antibody. The trial data showed that removab significantly increases time to tumor progression and has a positive influence on overall survival time. Moreover, a prolonged time interval between therapeutic punctures for the treatment of malignant ascites was seen in the removab group compared to the control group. This effect was also observed beyond the end of the study. In December 2006 and March 2007, Fresenius Biotech already announced encouraging results on the primary study endpoint – puncture free survival.
The submission for marketing authorization with the European Medicines Agency (EMEA) for removab in malignant ascites is expected in late 2007.
The Phase II studies with the antibody rexomun® to treat breast cancer and with the antibody removab to treat gastric cancer are ongoing. These studies started in March 2006 and June 2006, respectively. A phase II study with removab for the treatment of patients with ovarian cancer was started in Europe.
In the first half of 2007, Fresenius Biotech's operating income (EBIT) was € -20 million. For 2007, Fresenius Biotech expects an EBIT of approximately € -50 million (2006: € -45 million).
The Business Segments
Fresenius Medical Care
Fresenius Medical Care is the world's leading provider of services and products for patients with chronic kidney failure. As of June 30, 2007, Fresenius Medical Care was serving 171,687 patients in 2,209 dialysis clinics.


- Continued strong organic sales development
- Excellent earnings growth
- 2007 outlook raised
Fresenius Medical Care achieved significant sales growth of 21 % to US$ 4,725 million (H1 2006: US$ 3,912 million). This was mainly driven by the strong organic growth of 8 % and by the consolidation of Renal Care Group (RCG) as from the second quarter of 2006. Sales in dialysis care increased by 22 % to US$ 3,556 million (H1 2006: US$ 2,924 million). In dialysis products, Fresenius Medical Care achieved sales of US$ 1,169 million (H1 2006: US$ 988 million), an increase of 18 %.
In North America Fresenius Medical Care's sales increased by 20 % to US$ 3,297 million (H1 2006: US$ 2,754). Sales outside North America ("International") grew by 23 % (in constant currency: 16 %) to US$ 1,428 million (H1 2006: US$ 1,158 million). This was driven by the positive operating performance in Europe, the Asia-Pacific region and in Latin America.
Fresenius Medical Care increased EBIT by 23 % to US$ 756 million (H1 2006: US$ 616 million, adjusted US$ 581 million including a gain from the divestiture of US dialysis clinics and one-time expenses related to the RCG acquisition). The EBIT margin was 16.0 % (H1 2006: 15.7 %, adjusted 14.8 %). Net income increased by 38 % to US$ 339 million (H1 2006: US$ 246 million, including one-time expenses of US$ 16 million).
Based on the strong operational performance in the first half of 2007, Fresenius Medical Care raises its outlook for the full year 2007 and now expects to achieve revenue of more than US$ 9.5 billion. This represents an increase of at least 12 %. Previously, Fresenius Medical Care expected revenue of approximately US$ 9.4 billion. Net income is now projected to be in the range of US$ 685 million to US$ 705 million in 2007. This represents an increase of between 19 % and 23 % on an adjusted basis as compared to 2006 after one-time effects. On a reported basis, this translates into an increase in net income of between 28 % and 31 %. Previously, Fresenius Medical Care expected net income in the range of US$ 675 million to US$ 695 million.
For further information, please see Fresenius Medical Care's press release at www.fmc-ag.com.
Fresenius Kabi
Fresenius Kabi offers infusion therapies and clinical nutrition for seriously and chronically ill patients in the hospital and out-patient environments. The company is also a leading provider of transfusion technology products.

- Excellent organic sales growth
- Continued strong EBIT margin increase in second quarter and first half of 2007
- 2007 outlook fully confirmed
In the first half of 2007, Fresenius Kabi increased sales by 5 % to € 986 million (H1 2006: € 937 million). Currency translation effects had an impact of -2 %. This was mainly due to the depreciation of currencies in South Africa, China and Canada. Organic growth was 7 %. In the second quarter of 2007, Fresenius Kabi achieved strong organic growth of 8 %.
In Europe (excluding Germany) organic sales growth was 5 % while sales in Germany were at previous year's level. In the second quarter of 2007, organic sales growth was 1 % in Germany. Outside Europe, Fresenius Kabi achieved organic sales growth of 22 % in the Asia-Pacific region in the first half of 2007. Organic sales growth in Latin America was 10 % and in other regions 11 %.
Fresenius Kabi continued its excellent earnings growth. In the first half of 2007, EBIT increased by 14 % to € 159 million (H1 2006: € 139 million). The EBIT margin improved to 16.1 % (H1 2006: 14.8 %). In the second quarter of 2007, Fresenius Kabi achieved an EBIT margin of 16.3 %. Net income increased by 45 % to € 87 million (H1 2006: € 60 million, including one-time expenses for early debt refinancing of € 11 million).
Fresenius Kabi fully confirms its outlook for the full year 2007. Organic sales growth is projected to be 6 to 8 %. Continued strong sales growth is anticipated from the regions outside Europe. Based on the positive sales projection and further manufacturing and logistics improvements Fresenius Kabi expects an EBIT margin of 16.0 to 16.5 % in 2007.
Fresenius ProServe
Fresenius ProServe is a leading German hospital operator with 58 facilities. Moreover, the company offers engineering and services for hospitals and other health care facilities.

- Continued strong sales and earnings growth
- Sale of Pharmatec to Robert Bosch GmbH completed June 30, 2007
- 2007 earnings outlook raised
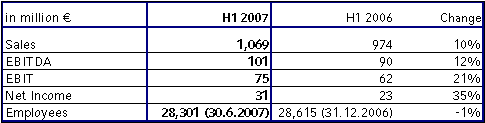
Fresenius ProServe's sales grew by 10 % to € 1,069 million (H1 2006: € 974 million). Organic sales growth was 2 %. EBIT increased by 21 % to € 75 million (H1 2006: € 62 million).
Sales in hospital operations (HELIOS Kliniken Group) grew by 16 % to € 890 million (H1 2006: € 767 million). The growth is mainly attributable to the acquisition of HUMAINE Kliniken, which was consolidated as from July 1, 2006. HELIOS achieved strong organic growth of 3 %. EBIT increased by 21 % to € 68 million, EBIT margin was 7.6 % (H1 2006: € 56 million and 7.3 %).
In the second quarter of 2007, HELIOS Kliniken inaugurated its newly constructed hospital Berlin-Buch. In parallel, HELIOS continued its growth strategy in the German hospital market. In July 2007, HELIOS agreed to acquire the Mariahilf hospital in Hamburg. The hospital has 255 beds and achieved revenues of € 26 million in 2006.
Sales in the engineering and services business was € 179 million (H1 2006: € 207 million). The decrease was mainly due to the sale of Pharmaplan, which was deconsolidated as of January 1, 2007. Organic growth was -2 %. EBIT was € 9 million (H1 2006: € 9 million).
Furthermore, Fresenius ProServe agreed to sell its subsidiary Pharmatec to Robert Bosch GmbH on May 1, 2007. In 2006, the company had sales of about € 30 million. The transaction was completed June 30, 2007.
Order intake in the engineering and services business was € 106 million (H1 2006: € 185 million). The decrease was due to the postponement of orders to the second half of 2007 and to a very strong order intake in the second quarter of 2006. Order backlog was € 379 million (December 31, 2006: € 428 million).
Based on the excellent financial results in the first half, Fresenius ProServe raises its 2007 EBIT outlook from previously € 160 to 170 million to now € ~170 million. The outlook for organic sales growth is confirmed at 2 to 3 %.
# # #
Conference call
As part of the publication of the results from the first half of 2007, a conference call will be held on August 2, 2007 at 2.00 p.m. CEDT (8.00 a.m. EDT). We invite all investors to follow the conference call over our web site under Investor Relations/Presentations. Following the conference, a recording of the call will be available.
Quarterly financial report
The report for the second quarter and the first half of 2007 will be available from August 14, 2007 under Investor Relations/Financial reports.
# # #
Fresenius is a health care group with international operations, providing products and services for dialysis, hospital and outpatient medical care. In 2006 group sales were approx. € 10.8 billion. On March 31, 2007 the Fresenius Group had 107,348 employees worldwide.
PDF-file includes:
Fresenius Group in Figures
- Consolidated statement of income (US GAAP) (unaudited)
- Key figures of the balance sheet (US GAAP) (unaudited)
- Cash flow statement (US GAAP) (unaudited)
- Segment reporting by business segment H1 2007 (US GAAP) (unaudited)
Having successfully completed its conversion into a European Company (SE) in July, Fresenius has now also given itself a new look.
The Group's corporate logo, colors and fonts have been revised and updated. The "F" in the new logo builds on the previous logo. Its form represents the Company's aspirations and continued growth. While the logo remained unchanged for over 13 years, Fresenius has grown significantly. Sales rose more than 10-fold from about € 1 billion in 1994 to € 10.8 billion in 2006, and the number of employees climbed from 8,000 in 1994 to about 109,000 this year.
Fresenius will continue to use its traditional blue color to represent the company, though in darker shade. The new corporate font "Interstate" is notable for its clarity. The business segments Fresenius Medical Care, Fresenius Kabi, Fresenius HELIOS and Fresenius VAMED will also review their corporate design.
You will find the new Fresenius company logo in print quality at http://www.fresenius.de/logos.
# # #
Fresenius is a health care group with international operations, providing products and services for dialysis, hospital and outpatient medical care. In 2006 group sales were approx. € 10.8 billion. On June 30, 2007 the Fresenius Group had 108,860 employees worldwide.
Summary Third Quarter 2007
- Net revenue : $ 2,426 million, + 9%
- Operating income (EBIT): $ 397 million, + 14%
- Net income: $ 181 million, + 30%
- Earnings per share: $ 0.61, + 29%
Summary First Nine Months 2007
- Net revenue : $ 7,151 million, + 16%
- Operating income (EBIT): $ 1,152 million, + 19%
- Net income: $ 520 million, + 35%
- Earnings per share: $ 1.76, + 34%
Fresenius Medical Care AG & Co. KGaA ("the Company"), the world's largest provider of Dialysis Products and Services, today announced its results for the third quarter and nine months of 2007.
Third Quarter 2007:
Revenue
Net revenue for the third quarter 2007 increased by 9% to $2,426 million (6% at constant currency) compared to the third quarter 2006. Organic revenue growth worldwide was 6%. Dialysis Services revenue grew by 6% to $1,801 million (4% at constant currency) in the third quarter of 2007. Dialysis Product revenue increased by 18% to $625 million (12% at constant currency) in the same period.
North America revenue increased by 3% to $1,660 million. Dialysis Services revenue grew by 1% to $1,494 million. Excluding effects of the divestiture of the perfusion business, Dialysis Service revenue increased by 3%. Average revenue per treatment for the U.S. clinics increased by 1% to $327 in the third quarter 2007 compared to $324 for the same quarter in 2006. Dialysis Product revenue increased by 18% to $167 million led by strong sales of our 2008K hemodialysis machines and the phosphate binding drug PhosLo.
International revenue was $766 million, an increase of 23% (14% at constant currency) compared to the third quarter of 2006. Dialysis Services revenue reached $307 million, an increase of 32% (23% at constant currency). Dialysis Product revenue rose by 18% to $459 million (9% at constant currency), led by strong sales of hemodialysis machines, peritoneal dialysis products and dialyzers.
Earnings
Operating income (EBIT) increased by 14% to $397 million compared to $349 million in the third quarter 2006. Operating income for the third quarter 2006 includes costs of $7 million related to costs of restructuring and a gain of $1 million from the divestiture of dialysis clinics in conjunction with the acquisition of Renal Care Group. Excluding these effects, operating income for the third quarter 2007 increased by 12%, resulting in an operating margin of 16.4%. For the third quarter 2006 the operating margin was 15.9%.
| Operating income (EBIT) before one-time items Three months ended September 30, (in US-$ million) |
2007 | 2006 | Growth |
| Operating income (EBIT) | 397 | 349 | + 14% |
| Cost of restructuring | - | 7 | |
| Gain from divestiture | - | (1) | |
| Operating income (EBIT) before one-time items | 397 | 355 | + 12% |
In North America, the operating margin increased from 16.3% (excluding the effects of one-time items) by 70 basis points to 17.0% due to revenue rate improvements, the new PhosLo business and higher product sales which more than offset higher personnel expenses. In the International segment, the operating margin decreased by 60 basis points to 17.6% mainly due to higher growth in the emerging markets and the effect of returning to normal plant maintenance from the shortened schedule in the prior year.
Net interest expense for the third quarter 2007 was $95 million compared to $100 million in the same quarter of 2006. This positive development was mainly attributable to a lower debt level in combination with lower average interest rates and the recognition of interest income related to the collection of overdue receivables. Interest expense was impacted by $5 million ($3 million, net after taxes) as a result of the write-off of deferred financing costs related to the repayment of a portion of the Company's senior credit agreement in connection with the issuance of $500 million senior notes.
Income tax expense was $115 million for the third quarter of 2007 compared to $105 million in the third quarter of 2006, reflecting effective tax rates of 38.0% and 42.3%, respectively. In the third quarter 2006, the tax rate had been impacted by a tax audit in Germany. Excluding this impact, the tax rate was at 39.1%.
Net income for the third quarter 2007 was $181 million, an increase of 30%. Net income increased by 27% when compared to the third quarter 2006 excluding the effects of one-time items in 2006.
| Net income before one-time items Three months ended September 30, (in US-$ million) |
2007 | 2006 | Growth |
| Net income | 181 | 139 | + 30% |
| Cost of restructuring | - | 5 | |
| Gain from divestiture | - | (1) | |
| Net income before one-time items | 181 | 143 | + 27% |
Earnings per share (EPS) for the third quarter of 2007 rose by 29% to $0.61 per ordinary share compared to $0.47 for the third quarter of 2006. The weighted average number of shares outstanding for the third quarter of 2007 was approximately 295.8 million shares compared to 294.5 million shares for the third quarter of 2006. The increase in shares outstanding resulted from stock option exercises in 2006 and in the first nine months of 2007.
Cash Flow
In the third quarter of 2007, the Company generated $382 million in cash from operations, representing approximately 16% of revenue. The strong cash flow generation was primarily supported by earnings and reduction of working capital.
A total of $123 million was spent for capital expenditures, net of disposals. Free Cash Flow before acquisitions was $259 million compared to $40 million in the third quarter of 2006 on a reported basis. A total of $24 million in cash was used for acquisitions, net of divestitures. Free Cash Flow after acquisitions was $235 million compared to $32 million in the third quarter of 2006.
Nine Months Ended September 30, 2007:
The operation of Renal Care Group (RCG) are included in the Company's consolidated statements of income and cash flows from April 1, 2006, therefore, the current results for the first nine months are not directly comparable with the results of the first nine months for 2006.
Revenue and Earnings
Net revenue for the nine months ended September 30, 2007 was $7,151 million, up 16% from the same period in 2006. At constant currency, net revenue rose by 14%. Organic growth was 7% in the first nine months of 2007.
Operating income (EBIT) increased by 19% to $1,152 million compared to $964 million in the first nine months of 2006. Operating income for the nine months ended September 30, 2006 includes costs of $12 million as a result of restructuring and the transformation of the Company's legal form, and a gain from the clinic divestitures of $40 million.
Excluding these items, operating income for the nine months 2007 increased by 23%. This performance resulted in an operating margin of 16.1% compared to 15.2% in the same period of 2006.
| Operating income (EBIT) before one-time items Nine months ended September 30, (in US-$ million) |
2007 | 2006 | Growth |
| Operating income (EBIT) | 1,152 | 964 | + 19% |
| Cost of restructuring and transformation | - | 12 | |
| Gain from divestiture | - | (40) | |
| Operating income (EBIT) before one-time items | 1,152 | 936 | + 23% |
Net interest expense for the nine months ended September 30, 2007 was $281 million compared to $255 million in the same period of 2006. The increase was mainly the result of additional interest expense partially offset by the write-off in 2006 of deferred financing costs related to the 2003 senior credit facility of $15 million, both in conjunction with the financing of the RCG acquisition.
Income tax expense was $331 million for the nine months compared to $314 million in the same period in 2006, reflecting tax rates of 38.0% and 44.3%, respectively. The tax rate for the first nine months of 2006 was impacted by tax payments in the U.S mainly related to the gain on the divestiture of dialysis clinics in the U.S. Excluding this impact, the effective tax rate for the first nine months 2006 was at 40.3%.
For the nine months ended September 30, 2007, net income was $520 million, up 35% from the same period in 2006. Net income for the nine months of 2007 increased by 28% compared to the same period 2006 excluding the effects of one-time items in 2006.
| Net income before one-time items Nine months ended September 30, (in US-$ million) |
2007 | 2006 | Growth |
| Net income | 520 | 385 | + 35% |
| Cost of restructuring and transformation | - | 7 | |
| Write-off FME prepaid financing fees | 9 | ||
| Loss from divestiture | - | 4 | |
| Net income before one-time items | 520 | 405 | + 28% |
For the nine months ended September 30, 2007, earnings per ordinary share rose by 34% to $1.76. The weighted average number of shares outstanding during the nine months 2007 was approximately 295.4 million.
Cash Flow
Cash from operations for the first nine months of 2007 was $890 million compared to $465 million for the same period in 2006 on a reported basis. Excluding the effects of one-time items, cash from operations was $663 million for nine months of 2006. The increase compared to prior year was mainly due to increased earnings and a reduction in working capital.
A total of $364 million was used for capital expenditures, net of disposals. Free Cash Flow before acquisitions for the nine months of 2007 was $526 million compared to $192 million in same period in 2006. The underlying Free Cash Flow before acquisitions and the effects of one-time items for the first nine months of 2006 was $390 million. A total of $110 million in cash was used for acquisitions, net of divestitures.
Please refer to the attachments for a complete overview on the third quarter and first nine months of 2007.
Patients – Clinics – Treatments
As of September 30, 2007, Fresenius Medical Care treated 172,227 patients worldwide, which represents a 7% increase in patients compared to last year. North America provided dialysis treatments for 120,607 patients, an increase of 3%. Including 33 clinics managed by Fresenius Medical Care North America, the number of patients in North America was 122,479. The International segment served 51,620 patients, an increase of 16% over last year.
As of September 30, 2007, the Company operated a total of 2,221 clinics worldwide. This is comprised of 1,591 clinics in North America, an increase of 3%, and 630 clinics in the International segment, an increase of 16%.
Fresenius Medical Care delivered approximately 19.6 million dialysis treatments worldwide during the first nine months of 2007. This represents an increase of 13% year over year. North America accounted for 13.7 million treatments, an increase of 11%, and the International segment delivered 5.9 million treatments, an increase of 16% over last year.
Employees
As of September 30, 2007, Fresenius Medical Care had 60,625 employees (full-time equivalents) worldwide compared to 56,803 employees at the end of 2006. The increase of 3,822 employees is primarily due to acquisitions in Asia and continued organic growth in the U.S.
Debt/EBITDA Ratio
The ratio of debt to Earnings before Interest, Taxes and Amortization (EBITDA) decreased from 3.44 at the end of the third quarter of 2006 to 2.88 at the end of the third quarter 2007. At the end of 2006, the debt/EBITDA ratio was 3.23.
Rating
There have been no ratings changes in the third quarter 2007, Standard & Poor's Ratings Services rates the Company's corporate credit rating as 'BB' with a ‘stable' outlook.
Moody's rates the Company's corporate credit rating as ‘Ba2' with a ‘positive' outlook.
Issuance of 10 Year Senior Notes
At the beginning of the third quarter 2007, Fresenius Medical Care issued Senior Notes due 2017 in the amount of $500 million. The coupon is 6 7/8%. Proceeds were used to reduce indebtedness under the Company's senior secured bank credit facility and other, short-term debt.
Outlook for 2007 Confirmed
The Company confirms its outlook for the full year 2007 and expects to achieve revenue of more than $9.5 billion. This represents an increase of at least 12%.
Net income was projected to be in the range of $685 million to $705 million in 2007. Based on the strong performance in the third quarter, the Company now expects the net income to be at the upper end of this guidance.
In addition, the Company still expects spending on capital expenditures and acquisitions to be approximately $650 million in 2007. The debt/EBITDA ratio is projected to be below 3.0 by the end of 2007.
For 2010, Fresenius Medical Care continues to expect revenue of more than $11.5 billion. Earnings after tax are projected to grow in the low- to mid-teens per year.
Ben Lipps, Chief Executive Officer of Fresenius Medical Care, commented: "Third quarter performance continues the strong trend of this year and was clearly outstanding considering the temporary US regulatory challenges. The North American segment achieved operating margins at the high end of its target. The International segment continues its strong double-digit revenue growth even during the Summer Quarter. We continued to grow above market in our product business on a worldwide basis. Cash flow from operations was very strong and clearly ahead of our expectations. We are clearly on track to achieve our targets for the current year and for 2010."
Video Webcast
Fresenius Medical Care will hold a press conference at its headquarters in Bad Homburg, Germany, to discuss the results of the third quarter and the first nine months of 2007 on October 31, 2007, at 10:00 a.m. CET. The Company cordially invites journalists to view the live video webcast of the meeting here on this website. A replay will be available shortly after the meeting.
# # #
Fresenius Medical Care is the world's largest integrated provider of products and services for individuals undergoing dialysis because of chronic kidney failure, a condition that affects more than 1,500,000 individuals worldwide. Through its network of 2,221 dialysis clinics in North America, Europe, Latin America, Asia-Pacific and Africa, Fresenius Medical Care provides dialysis treatment to 172,227 patients around the globe. Fresenius Medical Care is also the world's leading provider of dialysis products such as hemodialysis machines, dialyzers and related disposable products. Fresenius Medical Care is listed on the Frankfurt Stock Exchange (FME, FME3) and the New York Stock Exchange (FMS, FMS/P).
For more information about Fresenius Medical Care visit the Company's website at www.fmc-ag.com.
Fresenius Medical Care
Statement of Earnings
see PDF-file



