Fresenius Kabi today announced the immediate availability of two drugs in the United States. The first product, Ganirelix Acetate Injection, a generic fertility drug is part of the company’s expansion in women’s health. Ganirelix Acetate Injection is indicated for the inhibition of premature surges in luteinizing hormone, a chemical in the body that triggers reproductive processes such as ovulation. The second product is fentanyl citrate in a Simplist® ready-to-administer prefilled syringe presentation. The product is the only 100 mcg per 2 mL presentation available on the U.S. market in a manufacturer-prepared prefilled syringe.
Fresenius Kabi today announced the immediate availability of two drugs in the United States. The first product, Ganirelix Acetate Injection, a generic fertility drug is part of the company’s expansion in women’s health. Ganirelix Acetate Injection is indicated for the inhibition of premature surges in luteinizing hormone, a chemical in the body that triggers reproductive processes such as ovulation. The second product is fentanyl citrate in a Simplist® ready-to-administer prefilled syringe presentation. The product is the only 100 mcg per 2 mL presentation available on the U.S. market in a manufacturer-prepared prefilled syringe.
Under the U.S. Securities Act of 1933, as amended (the “Securities Act”), this press release may be deemed to be offering material of Fresenius Medical Care AG & Co. KGaA (“FME”). FME has filed a registration statement on Form F-4 under the Securities Act with the U.S. Securities and Exchange Commission (the “SEC”), including an information statement/prospectus constituting a part thereof. FME SHAREHOLDERS ARE URGED TO READ THE REGISTRATION STATEMENT AND ANY OTHER RELEVANT DOCUMENTS FILED OR THAT WILL BE FILED WITH THE SEC, INCLUDING THE INFORMATION STATEMENT/PROSPECTUS THAT IS PART OF THE REGISTRATION STATEMENT, AS THEY BECOME AVAILABLE, BECAUSE THEY CONTAIN OR WILL CONTAIN IMPORTANT INFORMATION ABOUT THE PROPOSED CONVERSION DESCRIBED THEREIN. The final information statement/prospectus has been distributed to FME shareholders. Shareholders may obtain a free copy of the disclosure documents and other documents filed by FME with the SEC at the SEC’s website at www.sec.gov or from Fresenius Medical Care AG & Co. KGaA, Attention: Investor Relations, Else-Kröner-Straße 1, 61352 Bad Homburg v.d.H., Germany.
Fresenius Medical Care, the world's leading provider of products and services for individuals with renal diseases has appointed Martin Fischer (46) as Chief Financial Officer as of October 1, 2023. He will succeed Helen Giza who was appointed as Chief Executive Officer and Chair of the Management Board in December 2022 and continues to serve as acting Chief Financial Officer, until her successor will join. Martin Fischer will be based in Bad Homburg, Germany and will assume responsibility for the Global Finance Organization of Fresenius Medical Care. Upon effectiveness of the Company’s proposed change of form from KGaA to German stock corporation, Martin Fischer will become a member of the Management Board of Fresenius Medical Care AG.
Martin Fischer has been Head of Finance for Siemens Healthineers Diagnostics Division based in Tarrytown, NY, U.S. since 2019. Previously, he headed the Board Office and Organizations function for Siemens Healthineers after leading the business plan and operating model development for the company’s initial public offering in March 2018. Prior to that, Fischer held a number of key international operational and finance positions in healthcare within Siemens AG. Martin Fischer holds a degree in business informatics from the Reutlingen University of Applied Sciences and an MBA from Friedrich Alexander University in Nuremberg. He completed the Chief Financial Officer Program at Columbia Business School in New York, USA.
Michael Sen, Chairman of the Supervisory Board of Fresenius Medical Care Management AG, says: "With Martin Fischer's appointment, we are strengthening a vital function in the Management Board of Fresenius Medical Care. Martin Fischer has a deep understanding of the international healthcare market, both from the U.S. perspective and out of Germany. That's a decisive advantage in getting the company back on track."
Helen Giza, CEO and Chair of the Management Board, said: "Martin Fischer has proven that he can successfully drive fundamental change in organizations. In our organizational transformation and turnaround management, we will benefit from his finance and healthcare expertise. Martin will be an important contributor to the execution of our strategy in unlocking value as the leading kidney care company."
Martin Fischer said: “There are great challenges ahead of us. I am convinced of the company's potential and look forward to realizing it together with the global team of Fresenius Medical Care. I am excited to bring my experience and expertise to support the company’s transformation.”
This release contains forward-looking statements that are subject to various risks and uncertainties. Actual results could differ materially from those described in these forward-looking statements due to various factors, including, but not limited to, changes in business, economic and competitive conditions, legal changes, regulatory approvals, impacts related to the COVID-19 pandemic results of clinical studies, foreign exchange rate fluctuations, uncertainties in litigation or investigative proceedings, and the availability of financing. These and other risks and uncertainties are detailed in Fresenius Medical Care AG & Co. KGaA's reports filed with the U.S. Securities and Exchange Commission. Fresenius Medical Care AG & Co. KGaA does not undertake any responsibility to update the forward-looking statements in this release.
Fresenius Kabi announced today the launch of its citrate-free biosimilar Idacio®* (adalimumab-aacf) for use in the treatment of chronic autoimmune diseases, in the U.S. Idacio® (adalimumab-aacf) is a tumor necrosis factor (TNF) blocker and a biosimilar to Humira®** (adalimumab). Idacio® is Fresenius Kabi’s second biosimilar launched in the U.S. and another key milestone in the company’s Vision 2026 growth strategy to broaden its biopharma global reach.
* Idacio® is a registered trademark of Fresenius Kabi Deutschland GmbH in selected countries
**Humira® is a registered trademark of AbbVie, Inc.
Fresenius Kabi announced today the launch of its citrate-free biosimilar Idacio®* (adalimumab-aacf) for use in the treatment of chronic autoimmune diseases, in the U.S. Idacio® (adalimumab-aacf) is a tumor necrosis factor (TNF) blocker and a biosimilar to Humira®** (adalimumab). Idacio® is Fresenius Kabi’s second biosimilar launched in the U.S. and another key milestone in the company’s Vision 2026 growth strategy to broaden its biopharma global reach.
* Idacio® is a registered trademark of Fresenius Kabi Deutschland GmbH in selected countries
**Humira® is a registered trademark of AbbVie, Inc.
- Dr. Ernst Wastler will leave the Fresenius Management Board upon reaching retirement age on July 18, 2023
- Changes and rejuvenation in the Management team of the Fresenius Vamed business segment
- Strengthened control function through new appointments in the reduced VAMED Supervisory Board and the establishment of an Audit Committee
- Following successful deconsolidation, Fresenius Medical Care will also no longer be represented on the Fresenius Board in future
- The composition of the Board reflects realignment through #FutureFresenius
The healthcare group Fresenius will have a revised Management team going forward. Dr. Ernst Wastler, previously responsible for Fresenius Vamed, will retire as Chairman of the VAMED Management Board and consequently from the Fresenius Management Board upon reaching retirement age on July 18, 2023. Dr. Klaus Schuster and Frank-Michael Frede will be appointed to the VAMED Management Board. Dr. Klaus Schuster will assume the new role of Spokesman of the VAMED Management Board but will not be represented on the Fresenius Management Board. Dr. Michael Moser, a new member of the Fresenius Management Board, will be responsible for Fresenius Vamed within the Fresenius Board.
Following the successful deconsolidation of Fresenius Medical Care, Helen Giza will also step down from the Fresenius Management Board. The #FutureFresenius strategy, with its realignment of business segments into operating and investment companies, is also reflected in the composition of the Fresenius Management Board.
"I would like to thank Dr. Wastler for his many years of highly dedicated work on the Fresenius Board," said Wolfgang Kirsch, Chairman of the Supervisory Board of Fresenius. "The leaner Fresenius Board in the future also takes into account the changes on the path to #FutureFresenius that Michael Sen and the Management Board team are successfully and consistently driving forward."
Dr. Klaus Schuster joined VAMED Management and Service GmbH as Chief Operating Officer (COO) in 2020. Schuster is a medical doctor and worked as a physician at Landesklinikum St. Pölten for ten years. He studied and obtained his doctorate at the Medical University of Vienna and holds an MBA in Health Care Administration from Danube University Krems.
Also appointed to the VAMED Board as of July 1, 2023, is Frank-Michael Frede, CEO of VAMED Deutschland Holding since 2022.
Gottfried Koos' (67) tenure on the VAMED Board will end on June 30, 2023. The four-member VAMED Board will continue to include the two current members, Andrea Raffaseder and Andreas Wortmann. Andreas Wortmann, Chief Financial Officer, will additionally take on the newly created role of Chief Transformation Officer.
Strengthened control function
The control function of the VAMED Supervisory Board will be strengthened. Firstly, it will be reduced from eight to six members. Commercial Councillor Karl Samstag, previously Deputy Chairman of the VAMED AG Supervisory Board and retired CEO of Austria Creditanstalt AG, as well as Dr. Robert Hink, former Secretary General of the Austrian Association of Municipalities, will resign from their positions with effect from the date of the next ordinary Supervisory Board meeting on July 12, 2023.
Dr. Dieter Schenk, Deputy Chairman of the Supervisory Board of Fresenius Management SE, will continue to lead the VAMED Supervisory Board. Sara Hennicken, CFO of Fresenius and a member of the VAMED Supervisory Board since December 2022, will remain a member of this Board and is due to be elected Deputy Chairman. Andreas Schmidradner, Advisor to the Management Board of B&C Industrieholding GmbH, will also continue to be a member of the Supervisory Board. Dr. Michael Moser, a future member of the Fresenius Management Board, was newly elected to the VAMED Supervisory Board with effect from July 12, 2023. Together with two employee representatives, Sara Hennicken, Dr. Dieter Schenk, Andreas Schmidradner and Dr. Michael Moser will form the six-member VAMED Supervisory Board going forward. Additionally, an Audit Committee consisting of Sara Hennicken as Chair, Michael Moser as Deputy Chair, and potentially one employee representative will be established.
This release contains forward-looking statements that are subject to various risks and uncertainties. Future results could differ materially from those described in these forward-looking statements due to certain factors, e.g. changes in business, economic and competitive conditions, regulatory reforms, results of clinical trials, foreign exchange rate fluctuations, uncertainties in litigation or investigative proceedings, the availability of financing and unforeseen impacts of international conflicts. Fresenius does not undertake any responsibility to update the forward-looking statements in this release.
- Dr. Ernst Wastler will leave the Fresenius Management Board upon reaching retirement age on July 18, 2023
- Changes and rejuvenation in the Management team of the Fresenius Vamed business segment
- Strengthened control function through new appointments in the reduced VAMED Supervisory Board and the establishment of an Audit Committee
- Following successful deconsolidation, Fresenius Medical Care will also no longer be represented on the Fresenius Board in future
- The compostition of the Board reflects realignment through #FutureFresenius
The healthcare group Fresenius will have a revised Management team going forward. Dr. Ernst Wastler, previously responsible for Fresenius Vamed, will retire as Chairman of the VAMED Management Board and consequently from the Fresenius Management Board upon reaching retirement age on July 18, 2023. Dr. Klaus Schuster and Frank-Michael Frede will be appointed to the VAMED Management Board. Dr. Klaus Schuster will assume the new role of Spokesman of the VAMED Management Board but will not be represented on the Fresenius Management Board. Dr. Michael Moser, a new member of the Fresenius Management Board, will be responsible for Fresenius Vamed within the Fresenius Board.
Following the successful deconsolidation of Fresenius Medical Care, Helen Giza will also step down from the Fresenius Management Board. The #FutureFresenius strategy, with its realignment of business segments into operating and investment companies, is also reflected in the composition of the Fresenius Management Board.
"I would like to thank Dr. Wastler for his many years of highly dedicated work on the Fresenius Board," said Wolfgang Kirsch, Chairman of the Supervisory Board of Fresenius. "The leaner Fresenius Board in the future also takes into account the changes on the path to #FutureFresenius that Michael Sen and the Management Board team are successfully and consistently driving forward."
Dr. Klaus Schuster joined VAMED Management and Service GmbH as Chief Operating Officer (COO) in 2020. Schuster is a medical doctor and worked as a physician at Landesklinikum St. Pölten for ten years. He studied and obtained his doctorate at the Medical University of Vienna and holds an MBA in Health Care Administration from Danube University Krems.
Also appointed to the VAMED Board as of July 1, 2023, is Frank-Michael Frede, CEO of VAMED Deutschland Holding since 2022.
Gottfried Koos' (67) tenure on the VAMED Board will end on June 30, 2023. The four-member VAMED Board will continue to include the two current members, Andrea Raffaseder and Andreas Wortmann. Andreas Wortmann, Chief Financial Officer, will additionally take on the newly created role of Chief Transformation Officer.
Strengthened control function
The control function of the VAMED Supervisory Board will be strengthened. Firstly, it will be reduced from eight to six members. Commercial Councillor Karl Samstag, previously Deputy Chairman of the VAMED AG Supervisory Board and retired CEO of Austria Creditanstalt AG, as well as Dr. Robert Hink, former Secretary General of the Austrian Association of Municipalities, will resign from their positions with effect from the date of the next ordinary Supervisory Board meeting on July 12, 2023.
Dr. Dieter Schenk, Deputy Chairman of the Supervisory Board of Fresenius Management SE, will continue to lead the VAMED Supervisory Board. Sara Hennicken, CFO of Fresenius and a member of the VAMED Supervisory Board since December 2022, will remain a member of this Board and is due to be elected Deputy Chairman. Andreas Schmidradner, Advisor to the Management Board of B&C Industrieholding GmbH, will also continue to be a member of the Supervisory Board. Dr. Michael Moser, a future member of the Fresenius Management Board, was newly elected to the VAMED Supervisory Board with effect from July 12, 2023. Together with two employee representatives, Sara Hennicken, Dr. Dieter Schenk, Andreas Schmidradner and Dr. Michael Moser will form the six-member VAMED Supervisory Board going forward. Additionally, an Audit Committee consisting of Sara Hennicken as Chair, Michael Moser as Deputy Chair, and potentially one employee representative will be established.
Please also read VAMED's press release. You will find it in the download area on the right, please use the second download link.
This release contains forward-looking statements that are subject to various risks and uncertainties. Future results could differ materially from those described in these forward-looking statements due to certain factors, e.g. changes in business, economic and competitive conditions, regulatory reforms, results of clinical trials, foreign exchange rate fluctuations, uncertainties in litigation or investigative proceedings, the availability of financing and unforeseen impacts of international conflicts. Fresenius does not undertake any responsibility to update the forward-looking statements in this release.

Fresenius has reached an important milestone in its IT transformation. The company successfully migrated its key SAP systems into the cloud with RISE with SAP, a comprehensive set of packages provided by SAP that helps companies transform into intelligent enterprises. This strategic move laid the foundation for future innovation targets and enables Fresenius to improve scalability, enhance application security and drive the digitalization of its global business processes.
The migration encompassed a wide range of systems, including e.g. ERP (Enterprise Resource Planning) systems for core business processes in finance, manufacturing, supply chain and procurement as well as CRM (Customer Relationship Management) systems, among others.
“The SAP RISE migration is accelerating our #FutureFresenius journey. The ability to scale our IT landscape more flexibly enables us to gain efficiency and to adapt to changes faster”, said Michael Sen, CEO Fresenius. “Digitizing our organization and the healthcare industry requires scalable platforms and working in ecosystems with internal and external partners. Digitization will be a key enabler for our business to advance patient care.”
“We are excited to partner with Fresenius on their digital transformation journey with RISE with SAP,” said Christian Klein, CEO and Member of the Executive Board of SAP SE. “Our solutions will empower Fresenius to streamline operations, enhance efficiency, and deliver even more exceptional value to its patients and customers.”
The migration project involved extensive collaboration between Fresenius and SAP where 29 system landscapes containing 134 systems have been migrated into the cloud smoothly and in record time of less than 15 months. The project's completion sets the stage for Fresenius to leverage the full potential of the latest SAP technologies, including SAP S/4HANA Cloud, to accelerate innovation and deliver exceptional value to the Fresenius Group.
The migration has resulted in improved security and resilience of all SAP systems. It allows the company to identify and resolve issues before they impact the business through delays or outages. The migrated systems show better performance in general.
“We have a long and successful history of working with SAP for 30 years now. We're thrilled with the outcome of the cloud migration. It is proof that all the hard work and close collaboration on challenges has paid off. The dedication, expertise, and collaboration of everyone involved has been instrumental in achieving this significant milestone. As we move forward, we're confident that the cloud transformation will empower our organization to innovate and excel in today's competitive healthcare market”, said Ingo Elfering, Fresenius Group CIO. “Migrating our core SAP databases to the cloud with RISE with SAP will also provide a secure and stable platform for our future SAP S/4HANA journey”, he added.
SAP S/4HANA is an integrated enterprise resource planning (ERP) with a focus on intelligent automation and easy-to-use interfaces, it helps companies achieve digital transformation by providing comprehensive solutions for finance, logistics, customer service, supply chain and more.
This release contains forward-looking statements that are subject to various risks and uncertainties. Future results could differ materially from those described in these forward-looking statements due to certain factors, e.g. changes in business, economic and competitive conditions, regulatory reforms, results of clinical trials, foreign exchange rate fluctuations, uncertainties in litigation or investigative proceedings, the availability of financing and unforeseen impacts of international conflicts.
Fresenius does not undertake any responsibility to update the forward-looking statements in this release.

Infusion Pumps
Drugs that are effective in even the smallest doses are often no longer administered using a needle. With syringe pumps, these highly effective substances can be administered via a vein over longer periods of time. Even the smallest quantities can be precisely dosed. Fresenius Kabi's medical devices are also used to administer infusion solutions. Syringe pumps are used for the precise administration of drugs even in very small quantities (0.1 to 200 milliliters per hour), while volumetric infusion pumps are used for the precise administration of larger quantities (1 to 1,500 milliliters per hour). Fresenius Kabi is among the leading manufacturers of pumps for infusion and clinical nutrition in Europe.
Nutrition Systems
Patients who are either unable to eat, prohibited from eating, or unwilling to eat require individualized nutritional therapy to prevent malnutrition and thereby influence the course of the disease in a positive way. Enteral nutrition is generally to be preferred if the function of the gastrointestinal tract is still intact and if there are no contraindications. The advantages of enteral nutrition therapy are the physiological routes of application, direct supply to the intestine, maintenance of the mucosa and barrier function, lower complication rates and high cost-effectiveness. Enteral nutrition can also be combined with parenteral nutrition for critically ill patients. To ensure that the required nutrients can be administered, Fresenius Kabi offers a wide range of medical products for both inpatient and outpatient care. Patients in intensive care, gastroenterology, surgery, oncology, and pediatrics can be individually supplied with latex- and plasticizer-free transnasal and percutaneous feeding tubes, nutrition pumps, pump and gravity transfer devices including all supplies.
The effectiveness of a nutrition therapy depends not only on the selection of suitable nutrition components and probe position, but also essentially on the dosage and speed of application as well as practical feasibility. For this purpose, Fresenius Kabi offers Amika® nutrition pumps, a reliable and user-friendly application technology for safe and effective nutrition therapy. The Amika® nutrition pumps feature a compact design for convenient use in both inpatient and mobile, outpatient settings. Sophisticated programming options allow a variety of dosage settings for continuous and intermittent application of tube feeding and fluids. Multiple alarm functions and the integrated connection for nurse call ensure safe and reliable application management. The Amika® pump range is complemented by customized technical accessories that further facilitate the handling and use of the feeding pumps.
Gastrointestinal application of nutrients utilizes and maintains physiological regulatory mechanisms of the body. For example, enteral nutrition provides for maintenance of the intestinal mucosa as a natural barrier to bacteria and stimulation of gastrointestinal hormones.
Because enteral nutrition, as opposed to parenteral nutrition, is generally the more physiological form of nutrient delivery, it should always be chosen as a priority for patients with a functioning gastrointestinal tract.
When selecting enteral access, a distinction is made between transnasal and percutaneous feeding tubes. The tube feeding can be gastric and/or intestinal.
Fresenius Kabi offers a complete range of feeding tubes for all indications, from transnasal single- and multi-lumen feeding tubes to percutaneous feeding tubes in the form of initial insertion and exchange systems, which use particularly tissue-friendly and radiopaque materials.
Please visit Clinical Nutrition for further information on parenteral nutrition.
Contact
Fresenius Kabi AG
Else-Kröner-Str. 1
61352 Bad Homburg
Germany
T: +49 6172 686-0
communication@fresenius-kabi.com
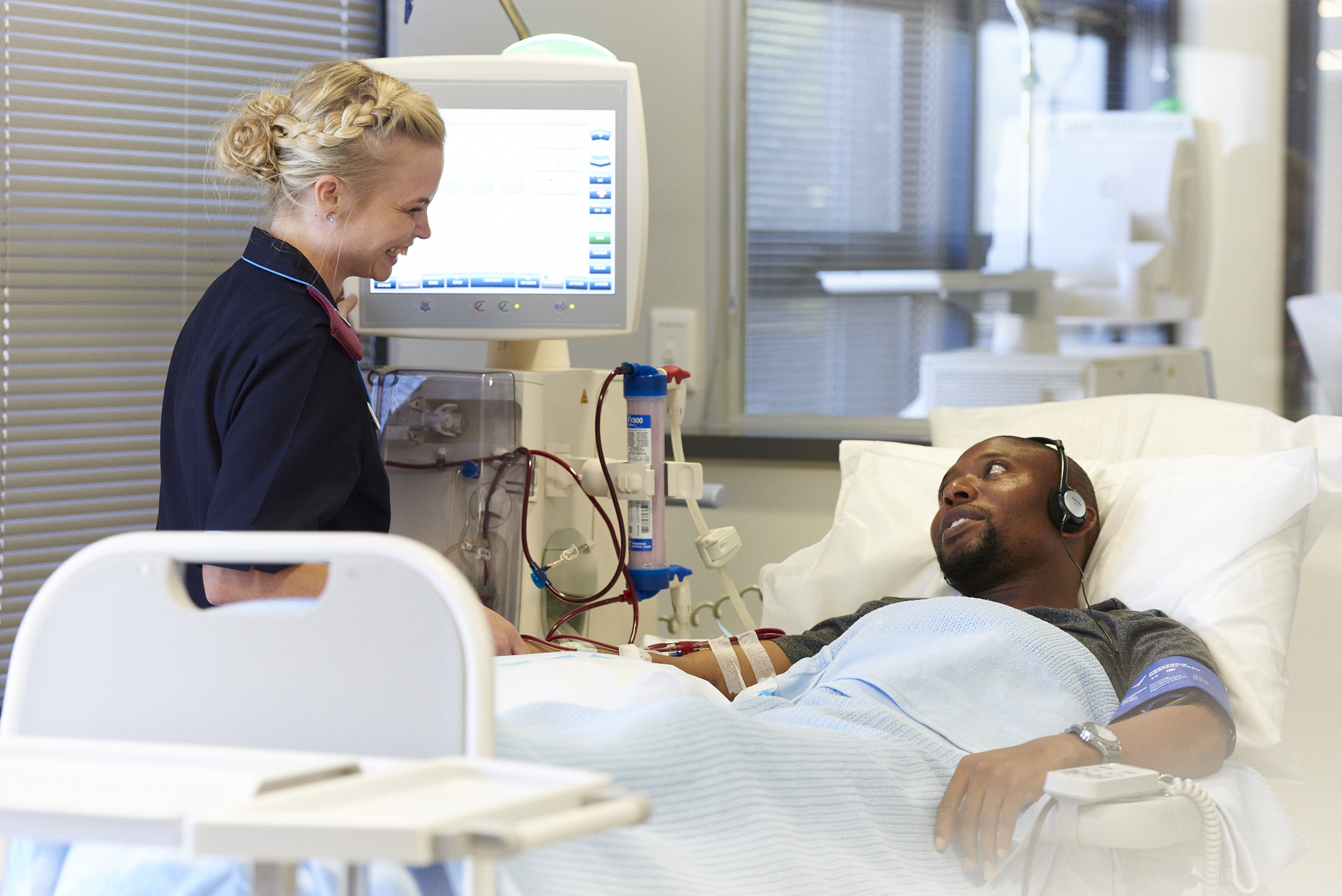
- The CONVINCE Trial study compares high-dose hemodiafiltration with standard, high-flux hemodialysis
- Study revealed a 23% decrease in mortality rates for patients treated with high-volume hemodiafiltration compared to those treated with more commonly used high-flux hemodialysis
- Exploration of methods to increase adoption of hemodiafiltration (HDF) making this therapeutic option accessible to patients
Fresenius Medical Care, the world's leading provider of products and services for individuals with renal diseases, participated in groundbreaking research that demonstrates that the mortality rate among kidney failure patients can be significantly reduced through the utilization of high-dose hemodiafiltration technology. Conducted by the CONVINCE consortium and led by the University Medical Center Utrecht, this international, randomized controlled trial marks a crucial milestone in comparing high-dose hemodiafiltration with standard, high-flux hemodialysis.
The study's findings reveal that patients treated with high-dose hemodiafiltration experienced a remarkable 23% decrease in mortality rates compared to those treated with the more commonly used high-flux hemodialysis. Hemodialysis represents the most prevalent form of dialysis for treating kidney failure. While techniques have improved over time, conventional high-flux hemodialysis primarily employs diffusion to remove small molecules and fluid from the blood. In contrast, high-dose hemodiafiltration incorporates both diffusion and convection techniques to eliminate larger molecules and effectively manage fluid replacement through convection.
Frank Maddux, MD, Chief Global Medical Officer of Fresenius Medical Care, expressed enthusiasm over the study's results, stating, "This meticulously designed and executed clinical study unquestionably demonstrates the efficacy of high volume hemodiafiltration for a significant portion of kidney failure patients. These findings have the potential to prompt significant changes in the standard treatment approach and, most importantly, contribute to reducing mortality rates among this vulnerable population in need of kidney replacement therapy."
Lead investigator Professor Peter Blankestijn from UMC Utrecht commented, "Our results unequivocally demonstrate the survival benefits of utilizing hemodiafiltration over hemodialysis in the treatment of kidney failure, akin to a remarkable 23% reduction in all-cause mortality. I am optimistic that hemodiafiltration can become the new standard of care.”
Chronic kidney disease poses a pressing global health challenge, affecting an estimated 830 million individuals worldwide, with nearly four million people reliant on dialysis. When the kidneys can no longer perform their vital functions, dialysis is employed to cleanse the blood by eliminating waste products and regulating fluid volume in the body.
The trial encompassed a total of 1,360 patients across 61 centers in eight European countries and followed up for a median of 30 months. Of these, 683 patients received high-dose hemodiafiltration and 677 received high-flux hemodialysis three times a week. The rate of mortality was 7.1 deaths per 100 patient-years in the group randomized to hemodiafiltration compared to 9.2 deaths per 100 patient-years in the hemodialysis group, corresponding to a relative reduction of 23%.
The CONVINCE trial was spearheaded by researchers from UMC Utrecht in collaboration with University College London (UCL), Charité Universitätsmedizin Berlin, University of Bari, The George Institute for Global Health, Imperial College London, and dialysis providers Fresenius Medical Care, Diaverum, and B. Braun Avitum.
While hemodialysis serves as the standard treatment in most countries, hemodiafiltration remains underutilized in certain regions and is yet to be adopted in places like the U.S. Fresenius Medical Care has taken the lead in developing machines that facilitate online fluid generation and innovative techniques for delivering hemodiafiltration.
"Fresenius Medical Care has a rich history of driving innovation in renal care, aimed at improving the lives of kidney disease patients worldwide," emphasized Frank Maddux, MD. "As pioneers in dialysis and membrane technologies, we will continue to explore methods that promote the adoption of hemodiafiltration, making this crucial therapeutic option more readily accessible to the patients we serve."
Disclaimer:
This release contains forward-looking statements that are subject to various risks and uncertainties. Actual results could differ materially from those described in these forward-looking statements due to various factors, including, but not limited to, changes in business, economic and competitive conditions, legal changes, regulatory approvals, impacts related to the COVID-19 pandemic results of clinical studies, foreign exchange rate fluctuations, uncertainties in litigation or investigative proceedings, and the availability of financing. These and other risks and uncertainties are detailed in Fresenius Medical Care AG & Co. KGaA's reports filed with the U.S. Securities and Exchange Commission. Fresenius Medical Care AG & Co. KGaA does not undertake any responsibility to update the forward-looking statements in this release.
__________
Notes to Editors:
Disclaimer: The information in this document is provided as is and no guarantee or warranty is given that the information is fit for any particular purpose. The user thereof uses the information at its sole risk and liability. The opinions expressed in the document are of the authors only and in no way reflect the European Commission’s opinions.
The CONVINCE study was exclusively supported by the European Commission Research & Innovation, Horizon 2020, Call H2020-SC1-2016-2017 under the topic SC1-PM-10-2017: Comparing the effectiveness of existing healthcare interventions in the adult population (grant no 754803).





