Fresenius Kabi, a business segment of health care group Fresenius, continues its growth strategy in intravenously administered drugs (i.v. drugs). The company today announced the acquisition of 73.3 % of the share capital of the Indian company Dabur Pharma Ltd. for a price of INR 8,782 million (€ 139 million) or INR 76.50 per share in cash. In accordance with Indian regulations, Fresenius Kabi also announced a public offer to acquire up to a further 20 % shareholding for a price of INR 76.50 per share in cash. Fresenius Kabi has entered into an agreement with a third party to secure the participation of 2.4 % of Dabur Pharma's share capital in the public offer.
Dabur Pharma, headquartered in New Delhi, is one of the leading suppliers of generic drugs and active pharmaceutical ingredients (API) to treat cancer. The company holds a substantial number of drug registrations in Asia, Europe and the US. Dabur Pharma is also one of the few manufacturers worldwide to hold international registrations for all steps within the manufacturing process of cytostatic agents. The company operates two production facilities in India and one in Great Britain as well as a research and development center equipped in accordance with European and US standards near New Delhi.
The acquisition significantly expands Fresenus Kabi's i.v. drug portfolio and secures its supply of high quality APIs for cytostatics. With the acquisition of Dabur Pharma, Fresenius Kabi will also broaden its offering of patient-specific oncology therapies. In future, Dabur Pharma will supply Fresenius Kabi's compounding centres in Europe, Asia-Pacific and Latin America where patient-specific formulations of i.v. drugs and parenteral nutrition are being prepared for cancer patients.
Dabur Pharma is listed on the Bombay Stock Exchange and on the National Stock Exchange of India. The company achieved sales of more than € 41 million with generic oncology drugs and APIs in fiscal year 2006/2007 (April 1, 2006 to March 31, 2007) and employs about 960 people. Dabur Pharma pursues an international growth strategy and will benefit from Fresenius Kabi's international sales and marketing organization going forward. In addition, Dabur Pharma will continue to be focused on expanding its research and development.
The acquisition will be entirely debt-financed from funds that have already been procured. The transaction is expected to be accretive to Fresenius Group's Cash EPS in 2 - 3 years.
Closing of the transaction is subject to completion of the public offer process in line with local regulations as well as relevant approvals required under Indian law. This is expected to occur at the beginning of Q3 2008.
Conference Call
A conference call to inform about the acquisition will be held on April 21, 2008 at 4.00 p.m. CEDT. You are cordially invited to follow the conference call in a live broadcast over the Internet at www.fresenius.com / Investor Relations / Presentations. A replay of the call will be available on our website shortly after the call.
Group Cash EPS: before transaction-related amortization of intangible assets.
About Fresenius SE
Fresenius is a health care group with international operations, providing products and services for dialysis, hospital and outpatient medical care. In 2007, group sales were approx. € 11.4 billion. On December 31, 2007 the Fresenius Group had 114,181 employees worldwide.
About Fresenius Kabi
Fresenius Kabi is the leader in infusion therapy and clinical nutrition in Europe and in its most important countries of Latin America and Asia Pacific. Fresenius Kabi's core product range includes infusion solutions for fluid substitution, blood volume expansion and parenteral nutrition, as well as products for enteral nutrition. Furthermore, the company provides concepts for ambulatory health care and is focused on managing and providing home therapies. With its philosophy "Caring for life" and a comprehensive product portfolio, the company aims at improving the quality of life of patients all over the world. On December 31, 2007 the company had 16,964 employees. In 2007, Fresenius Kabi achieved sales of € 2,030 million and an operating profit of € 332 million. Fresenius Kabi AG is a 100 % subsidiary of the health care group Fresenius SE.
About Dabur
Dabur Pharma Ltd. is committed to the discovery, development and marketing of drugs that fight cancer. Dedicated to its mission of making cancer therapy available to more and more people, it has been expanding ever since inception. The company is the leader in the Indian oncology market and it offers a complete range of products in this segment spanning across injectables, orals, intermediates and APIs and is present in over 40 countries. As of March 31, 2008, the company has 156,669,800 shares outstanding.
This release contains forward-looking statements that are subject to various risks and uncertainties. Future results could differ materially from those described in these forward-looking statements due to certain factors, e.g. changes in business, economic and competitive conditions, regulatory reforms, results of clinical trials, foreign exchange rate fluctuations, uncertainties in litigation or investigative proceedings, and the availability of financing. Fresenius does not undertake any responsibility to update the forward-looking statements in this release.
Summary First Quarter 2008:
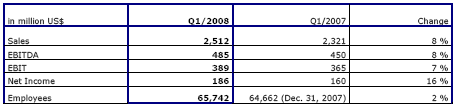
- Net revenue: $ 2,512 million, + 8%
- Operating income (EBIT): $ 389 million, + 7%
- Net income: $ 186 million, + 16%
- Earnings per share: $ 0.63, + 15%
Fresenius Medical Care AG & Co. KGaA ("the Company"), the world's largest provider of Dialysis Products and Services, today announced its results for the first quarter of 2008.
Revenue
Net revenue for the first quarter 2008 increased by 8% to $2,512 million (4% at constant currency) compared to the first quarter 2007. Organic revenue growth worldwide was 5%. Dialysis Services revenue grew by 5% to $1,844 million (3% at constant currency) in the first quarter of 2008. Dialysis Product revenue increased by 19% to $667 million (10% at constant currency) in the same period.
North America revenue increased by 2% to $1,668 million. Dialysis Services revenue grew by 1% to $1,495 million. Excluding effects of the divestiture of the perfusion business in spring 2007, Dialysis Service revenue increased by 3%. Average revenue per treatment for the U.S. clinics was at $326 in the first quarter 2008 compared to $329 for the first quarter of 2007 and $325 for the fourth quarter of 2007. Versus the fourth quarter of 2007, this development was based on an increase in underlying reimbursement rates and an increase in EPO utilization. Dialysis Product revenue increased by 12% to $172 million well above market and was led by strong sales of all of our major products, the 2008K hemodialysis machines, concentrates, dialyzers and the phosphate binding drug PhosLo.
International revenue was $844 million, an increase of 23% (10% at constant currency) compared to the first quarter of 2007. Dialysis Services revenue reached $349 million, an increase of 26% (13% at constant currency). Dialysis Product revenue rose by 22% to $495 million (9% at constant currency), led by strong dialyzer and dialysis machine sales.
Earnings
Operating income (EBIT) increased by 7% to $389 million compared to $365 million in the first quarter 2007. Operating margin decreased from 15.7% in the first quarter of 2007 to 15.5% in the first quarter of 2008 reflecting mainly the increased expenditures for our corporate research and development activities and the expansion in the International dialysis service business.
In North America, the operating margin increased by 60 basis points from 15.8% to 16.4% in the first quarter of 2008. The strong underlying business was supported by the increase in underlying reimbursement rates, dialysis services cost containment and a continued strong performance of renal products and PhosLo. This was partially offset by a lower utilization and reduced reimbursement rates for EPO.
In the International segment, the operating margin decreased by 60 basis points to 17.0% mainly due to the growth in the dialysis care business through an increased number of De Novo clinics and associated start-up costs.
Net interest expense for the first quarter 2008 was $83 million compared to $95 million in the same quarter of 2007. This positive development was mainly attributable to lower average interest rates.
Income tax expense was $114 million for the first quarter of 2008 compared to $103 million in the first quarter of 2007, reflecting effective tax rates of 37.3% and 38.0%, respectively.
Net income for the first quarter 2008 was $186 million, an increase of 16%.
Earnings per share (EPS) for the first quarter of 2008 rose by 15% to $0.63 per ordinary share compared to $0.54 for the first quarter of 2007. The weighted average number of shares outstanding for the first quarter of 2008 was approximately 296.6 million shares compared to 295.2 million shares for the first quarter of 2007. The increase in shares outstanding resulted from stock option exercises in 2007 and in the first quarter 2008.
Cash Flow
In the first quarter of 2008, the Company generated $192 million in cash from operations, representing approximately 8% of revenue. The cash flow generation was primarily affected by an increase in Days Sales Outstanding (DSO) in the first quarter of 2008 compared to 2007 and higher income tax payments.
A total of $154 million was spent for capital expenditures, net of disposals. Free Cash Flow before acquisitions was $38 million compared to $174 million in the first quarter of 2007 on a reported basis. A total of $32 million in cash was used for acquisitions net of divestitures. Free Cash Flow after acquisitions and divestitures was $6 million compared to $84 million in the first quarter last year.
Please refer to the appendix for a complete overview on the first quarter of 2008.
Patients – Clinics – Treatments
As of March 31, 2008, Fresenius Medical Care treated 177,059 patients worldwide, which represents a 5% increase in patients compared to the same period last year. North America provided dialysis treatments for 122,691 patients, an increase of 3%. Including 32 clinics managed by Fresenius Medical Care North America, the number of patients in North America was 124,403. The International segment served 54,368 patients, an increase of 8% over last year.
As of March 31, 2008, the Company operated a total of 2,297 clinics worldwide. This is comprised of 1,640 clinics in North America, an increase of 4%, and 657 clinics in the International segment, an increase of 6%.
Fresenius Medical Care delivered approximately 6.72 million dialysis treatments worldwide during the first quarter of 2008. This represents an increase of 5% year over the same quarter last year. North America accounted for 4.65 million treatments, an increase of 4%, and the International segment delivered 2.08 million treatments, an increase of 8% over last year.
Employees
As of March 31, 2008, Fresenius Medical Care had 62,504 employees (full-time equivalents) worldwide compared to 61,406 employees at the end of 2007. The increase of approximately 1,100 employees is primarily due to the Company's overall growth in business.
Debt/EBITDA Ratio
The ratio of debt to Earnings before Interest, Taxes and Amortization (EBITDA) decreased from 3.09 at the end of the first quarter 2007 to 2.82 at the end of the first quarter 2008. At the end of 2007, the debt/EBITDA ratio was 2.84.
Rating
Standard & Poor's has assigned upgraded debt and recovery ratings to the Company's unsecured debt issues in the first quarter of 2008. The Company's corporate credit rating as 'BB' with a ‘stable' outlook remained unchanged.
Moody's continued to rate the Company's corporate credit rating as ‘Ba2' with a ‘positive' outlook in the first quarter of 2008.
Outlook for 2008 fully confirmed
For the full year 2008, the Company confirms its outlook and expects to achieve revenue of more than $10.4 billion, an increase of more than 7%.
Net income is projected to be between $805 million and $825 million in 2008, an increase of 12% to 15%.
In addition, the Company expects to spend $650 to $750 million on capital expenditures and $150 to $250 million on acquisitions. The debt/EBITDA ratio is projected to decrease below 2.8 by the end of 2008.
For 2010, Fresenius Medical Care continues to expect revenue of more than $11.5 billion. Earnings after tax are projected to grow in the low- to mid-teens per year.
Ben Lipps, Chief Executive Officer of Fresenius Medical Care, commented: "We are pleased to have a strong start into the year, which is fully in line with our expectations and guidance. We have made good progress on our growth initiatives and continue to expand our global products and services presence. We also have expanded our corporate research activities that will benefit our future. Our new product launches in International and North America, along with the ongoing capacity expansions, provide the basis for the continued growth. We also expect the trend of anemia outcomes in the U.S. to improve further. We are confident to achieve our targets for 2008."
Conference Call
Fresenius Medical Care will hold a conference call to discuss the results of the first quarter of 2008 on Wednesday, April 30, 2008, at 3.30 p.m. CEDT / 9.30 a.m. EDT. The Company invites all journalists to listen to the live webcast of the meeting at the Company's website www.fmc-ag.com in the section "Investor Relations" / presentations. A replay will be available shortly after the meeting.
Fresenius Medical Care is the world's largest integrated provider of products and services for individuals undergoing dialysis because of chronic kidney failure, a condition that affects more than 1,600,000 individuals worldwide. Through its network of 2,297 dialysis clinics in North America, Europe, Latin America, Asia-Pacific and Africa, Fresenius Medical Care provides dialysis treatment to 177,059 patients around the globe. Fresenius Medical Care is also the world's leading provider of dialysis products such as hemodialysis machines, dialyzers and related disposable products. Fresenius Medical Care is listed on the Frankfurt Stock Exchange (FME, FME3) and the New York Stock Exchange (FMS, FMS/P).
For more information about Fresenius Medical Care visit the Company's website at www.fmc-ag.com.
This release contains forward-looking statements that are subject to various risks and uncertainties. Actual results could differ materially from those described in these forward-looking statements due to certain factors, including changes in business, economic and competitive conditions, regulatory reforms, foreign exchange rate fluctuations, uncertainties in litigation or investigative proceedings, and the availability of financing. These and other risks and uncertainties are detailed in Fresenius Medical Care AG & Co. KGaA's reports filed with the U.S. Securities and Exchange Commission. Fresenius Medical Care AG & Co. KGaA does not undertake any responsibility to update the forward-looking statements in this release.
Fresenius Medical Care
Statement of Earnings
see PDF-file
- Sales € 2.8 billion, +1 % at actual rates, +8 % in constant currency
- EBIT € 377 million, -1 % at actual rates, +7 % in constant currency
- Net income € 100 million, +8 % at actual rates, +13 % in constant currency
- Excellent growth in constant currency
- Currency impact based on translational effects
- Significant progress in generic I.V. drug strategy
- All business segments fully on track - Guidance for 2008 confirmed
Outlook for 2008 confirmed
Based on the Group's strong first quarter financial results Fresenius fully confirms its positive outlook for 2008: Group sales are expected to grow by 8 to 10 % in constant currency. Net income is expected to increase by 10 to 15 % in constant currency. All business segments are expected to contribute to this growth.
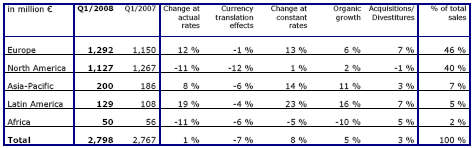
Sales growth of 8 % in constant currency
Group sales increased by 8 % in constant currency and by 1 % at actual rates to € 2,798 million (Q1 2007: € 2,767 million). Organic sales growth was 5 %. Acquisitions contributed a further 4 %. Divestitures reduced sales growth by 1 %. Currency translation had a negative impact of 7 %. This is mainly attributable to the average US dollar/Euro rate depreciating 14 % from Q1 2007.
Sales growth in the business segments was as follows:

In Europe sales grew by 13 % in constant currency with organic sales growth contributing 6 %. In North America sales grew by 1 % in constant currency. Organic growth was 2 %. Strong growth rates were achieved in the emerging markets with organic growth of 11 % in Asia-Pacific and 16 % in Latin America.
Excellent net income growth
Group EBITDA increased by 8 % in constant currency and by 1 % at actual rates to € 483 million (Q1 2007: € 479 million). Group operating income (EBIT) grew by 7 % in constant currency and decreased by 1 % at actual rates to € 377 million (Q1 2007: € 380 million). The Group's EBIT margin was 13.5 % (Q1 2007: 13.7 %).
Group net interest decreased to € -84 million (Q1 2007: € -95 million). This is mainly attributable to lower average interest rates at Fresenius Medical Care and currency translation effects.
The Group tax rate was 35.2 % (Q1 2007: 36.1 %).
Minority interest increased slightly to € 90 million (Q1 2007: € 89 million), of which 92 % was attributable to the minority interest in Fresenius Medical Care.
Group net income grew by 13 % in constant currency and by 8 % at actual rates to € 100 million (Q1 2007: € 93 million). Earnings per ordinary share and per preference share were € 0.64 (Q1 2007: ordinary share € 0.60, preference share € 0.60). This represents an increase of 7 % for both share classes.
Substantial investments in growth
Fresenius Group spent € 155 million for property, plant and equipment and intangible assets (Q1 2007: € 140 million). Acquisition spending was € 215 million (Q1 2007: € 155 million).
Sustainable cash flow development
Operating cash flow was € 278 million (Q1 2007: € 287 million). The cash flow margin was 9.9 % (Q1 2007: 10.4 %). Given increased net capital expenditure of € 162 million (Q1 2007: € 132 million), cash flow before acquisitions and dividends was € 116 million (Q1 2007: € 155 million). Free cash flow after net acquisitions (€ 158 million) and dividends (€ 5 million) was € -47 million (Q1 2007: € 88 million).
Solid balance sheet
Fresenius Group's total assets increased by 3 % in constant currency and decreased by 1 % at actual rates to € 15,149 million (December 31, 2007: € 15,324 million). Current assets increased by 4 % in constant currency and by 1 % at actual rates to € 4,319 million (December 31, 2007: € 4,291 million). Non-current assets were € 10,830 million (December 31, 2007: € 11,033 million).
Shareholders' equity including minority interest increased by 3 % in constant currency and decreased by 1 % at actual rates to € 5,988 million (December 31, 2007: € 6,059 million). The equity ratio (including minority interest) was 39.5 % (December 31, 2007: 39.5 %).
Group debt decreased by 2 % at actual rates to € 5,598 million (December 31, 2007: € 5,699 million). In constant currency, Group debt increased by 2 %. As of March 31, 2008, the net debt/EBITDA ratio was 2.6 (December 31, 2007: 2.6).
Number of employees increased
As of March 31, 2008, Fresenius increased the number of its employees by 2 % to 116,203 (December 31, 2007: 114,181). The growth was mainly attributable to Fresenius Kabi und Fresenius Medical Care.
Fresenius Biotech
Fresenius Biotech develops innovative therapies with trifunctional antibodies for the treatment of cancer. In the field of polyclonal antibodies, Fresenius Biotech has successfully marketed ATG-Fresenius S for many years. ATG-Fresenius S is an immunosuppressive agent used to prevent and treat graft rejection following organ transplantation.
Studies with the antibodies Removab® and Rexomun® in various indications are ongoing in Europe and the US.
Fresenius Biotech's EBIT was € -9 million (Q1 2007: € -11 million). For 2008, Fresenius Biotech expects an EBIT of approximately € -50 million.
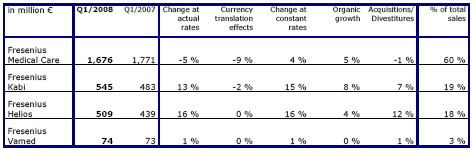
The Business Segments
Fresenius Medical Care
Fresenius Medical Care is the world's leading provider of services and products for patients with chronic kidney failure. As of March 31, 2008, Fresenius Medical Care was treating 177,059 patients in 2,297 dialysis clinics.
Strong start into the year - in line with expectations
Outlook 2008 fully confirmed
Fresenius Medical Care achieved sales growth of 8 % to US$ 2,512 million (Q1 2007: US$ 2,321 million). Organic growth was 5 %. Currency translation effects had a positive impact of 4 %. Sales in dialysis care increased by 5 % to US$ 1,844 million (Q1 2007: US$ 1,760 million). In dialysis products, sales grew by 19 % to US$ 667 million (Q1 2007: US$ 560 million).
In North America sales increased by 2 % to US$ 1,668 million (Q1 2007: US$ 1,637 million). Dialysis services revenue increased by 1 % (3 % adjusted for the divestiture of the perfusion business in spring 2007) to US$ 1,495 million. Average revenue per treatment in the US was US$ 326 in the first quarter (Q4 2007: US$ 325), based on an increase in underlying reimbursement rates and an increase in EPO utilization. Sales outside North America ("International" segment) grew by 23 % (10 % in constant currency) to US$ 844 million (Q1 2007: US$ 684 million). Strong sales growth in constant currency was achieved in Europe (+11 %) and Latin America (+14 %).
EBIT rose by 7 % to US$ 389 million (Q1 2007: US$ 365 million) resulting in an EBIT margin of 15.5 % (Q1 2007: 15.7 %). This reflects the increased expenditures for corporate research and development activities and the expansion in the International dialysis services business. The EBIT margin in North America increased by 60 basis points to 16.4 %, supported by improved underlying reimbursement rates, dialysis services cost containment and a continued strong performance of renal products and PhosLo. In the International segment, the EBIT margin decreased by 60 basis points to 17.0 % mainly due to the growth in the dialysis care business through an increased number of De Novo clinics and associated start-up costs.
Net income increased by 16 % to US$ 186 million (Q1 2007: US$ 160 million).
For 2008, Fresenius Medical Care fully confirms its outlook and expects to achieve revenue of more than US$ 10.4 billion, an increase of more than 7 %. Net income is expected to be between US$ 805 million and US$ 825 million, an increase of 12 % to 15 %.
For further information, please see Fresenius Medical Care's press release at www.fmc-ag.com.
Fresenius Kabi
Fresenius Kabi offers infusion therapies and clinical nutrition for seriously and chronically ill patients in the hospital and out-patient environments. The company is also a leading provider of transfusion technology products.
Excellent sales growth of 15 % in constant currency
Outlook 2008 fully confirmed
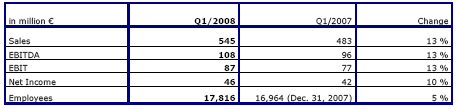
Fresenius Kabi increased sales by 15 % in constant currency and by 13 % at actual rates to € 545 million (Q1 2007: € 483 million). Organic growth was excellent at 8 %. Net acquisitions contributed a further 7 % to sales. Currency translation effects had a negative impact of 2 %. This was mainly due to the depreciation of currencies in Great Britain, South Africa and China.
Organic sales growth in Europe (excluding Germany) was 6 %. In Germany organic sales growth was 1 %. In the Asia-Pacific region Fresenius Kabi again achieved significant organic sales growth of 28 %. Organic sales growth in Latin America was 10 % and in other regions 6 %.
EBIT grew by 13 % to € 87 million (Q1 2007: € 77 million). The EBIT margin was 16.0 % (Q1 2007: 15.9 %). Net income grew by 10 % to € 46 million (Q1 2007: € 42 million).
Fresenius Kabi fully confirms the outlook for 2008: The company targets sales growth in constant currency of 12 to 15 %. Organic growth is expected to contribute around 7 % to this target. Further, Fresenius Kabi forecasts an EBIT margin of around 16.5 %.
On April 20, 2008, Fresenius Kabi announced the acquisition of 73.3 % of the share capital of the Indian company Dabur Pharma Ltd. Dabur is a leading supplier of oncology generics. With this acquisition, Fresenius Kabi strengthens its position in I.V. drugs. Dabur achieved sales of more than € 41 million with generic oncology drugs and APIs in fiscal year 2006/2007 (April 1, 2006 to March 31, 2007).
Fresenius Helios
Fresenius Helios is one of the largest private hospital operators in Germany. The HELIOS Kliniken Group owns 60 hospitals, including five maximum care hospitals in Berlin-Buch, Erfurt, Krefeld, Schwerin and Wuppertal. HELIOS treats about 500,000 inpatients per year at its clinics and has a total of approximately 17,400 beds.
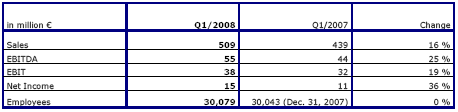
Positive financial performance of established clinics; Krefeld and Hüls with expected negative earnings contribution
Outlook 2008 fully confirmed
Fresenius Helios increased sales by 16 % to € 509 million (Q1 2007: € 439 million). Acquisitions contributed 11 % to overall sales growth. Organic growth was 4 %.
EBIT grew strongly by 19 % to € 38 million (Q1 2007: € 32 million) due to the very good financial performance of the established clinics. The first-time consolidation of HELIOS Klinikum Krefeld and the HELIOS Klinik Hüls had the expected negative impact on earnings. Nevertheless, the EBIT margin increased to 7.5 % (Q1 2007: 7.3 %). Net income improved by 36 % to € 15 million (Q1 2007: € 11 million).
Sales at the established clinics rose by 4 % to € 461 million. EBIT improved by 31 % to € 42 million. The EBIT margin was 9.1 % (Q1 2007: 7.3 %). The acquired clinics (consolidation < 1 year) achieved sales of € 48 million and an EBIT of € -4 million.
Fresenius Helios fully confirms its outlook for 2008: The company expects to achieve sales of more than € 2,050 million. EBIT is projected to increase to € 160 to 170 million, including the negative contribution of the clinics in Krefeld and Hüls.
Fresenius Vamed
Fresenius Vamed offers engineering and services for hospitals and other health care facilities.
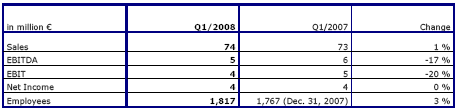
Sales and earnings performance fully in line with expectations
Outlook 2008 fully confirmed
In the first quarter of 2008, Fresenius Vamed achieved sales growth of 1 % to € 74 million (Q1 2007: € 73 million). The project business generated sales of € 35 million (Q1 2007: € 37 million), sales in the service business were € 39 million (Q1 2007: € 36 million).
EBIT was € 4 million (Q1 2007: € 5 million). The EBIT margin was 5.4 % (Q1 2007: 6.8 %). Net income was € 4 million (Q1 2007: € 4 million).
Order intake in the project business grew strongly by 89 % to € 125 million (Q1 2007: € 66 million). This was driven by obtaining the order for the planning and construction of the Tauern Spa World in Kaprun/Austria of about € 80 million. Fresenius Vamed will be also responsible for the operational management of Tauern Spa World following the completion of the project. Order backlog as of March 31, 2008 reached a new all-time high of € 595 million, an increase of 17 % (December 31, 2007: € 510 million).
Fresenius Vamed fully confirms its outlook for 2008 and expects to grow both sales and EBIT by 5 to 10 %.
Analyst Conference Call and Audio Webcast
As part of the publication of the results for the first quarter of 2008, a conference call will be held on April 30, 2008 at 2.00 p.m. CEDT (8.00 a.m. EDT). You are cordially invited to follow the conference call in a live broadcast over the Internet at www.fresenius.com / Investor Relations / Presentations. Following the call, a recording will be available.
Fresenius is a health care group with international operations, providing products and services for dialysis, hospital and outpatient medical care. In 2007, group sales were approx. € 11.4 billion. On March 31, 2008 the Fresenius Group had 116,203 employees worldwide.
For more information visit the Company's website at www.fresenius.com.
This release contains forward-looking statements that are subject to certain risks and uncertainties. Future results could differ materially from those described in these forward-looking statements due to various factors, e.g., changes in the business, economic and competitive environment, regulatory reforms, results of clinical trials, foreign exchange rate fluctuations, uncertainties in litigation or investigative proceedings, and the availability of financing. Fresenius does not undertake any responsibility to update the forward-looking statements in this release.
Board of Management: Dr. Ulf M. Schneider (President and CEO), Rainer Baule, Dr. Francesco De Meo, Dr. Jürgen Götz, Dr. Ben Lipps, Stephan Sturm, Dr. Ernst Wastler
Supervisory Board: Dr. Gerd Krick (Chairman)
Registered Office: Bad Homburg, Germany/Commercial Register No. HRB 10660
Fresenius Group in Figures
- Consolidated statement of income (US GAAP) (unaudited)
- Key figures of the balance sheet (US GAAP) (unaudited)
- Cash flow statement (US GAAP) (unaudited)
- Segment reporting by business segment Q1/2008 (US GAAP) (unaudited)
see PDF-file
Following an excellent year in 2007 and a strong first quarter, Fresenius Medical Care's Chief Executive Officer Ben Lipps today confirmed the outlook for the remainder of 2008. At the Annual General Meeting in Frankfurt, he said the Company expects to achieve revenues of more than $10.4 billion and earnings after tax between $805 and $825 million. Based on the strong revenue development and the Company's growth opportunities, Fresenius Medical Care also expects to meet its long-term target of more than $11.5 billion in revenues by 2010, and sustainable growth in earnings after tax in the low- to mid-teens per year.
"In 2007 we continued to expand our global presence," Ben Lipps said. "We invested in our production facilities in the United States and Germany, and acquired a production plant in China to meet the growing demand for our renal products. In Germany, we created more than 450 jobs in 2007 and the first quarter of 2008, primarily at our production sites in Schweinfurt and St. Wendel. The number of employees in Germany increased by about 16%. Within the next two years, we expect to increase our dialyzer production capacity from 75 million in 2007 to more than 90 million," Ben Lipps stated.
The new 5008S dialysis machine, which was introduced recently at the EDTA nephrological congress in Stockholm (Sweden), should also contribute to continued growth. The 5008S has been tested extensively around the world, and maintains the essential features of the 5008 system. The 5008S is designed to make online hemodiafiltration – currently the most advanced therapy – available to an increasing number of patients in selected markets.
During the Annual General Meeting, the shareholders approved the eleventh consecutive dividend increase with a large majority of 99.96%. The dividend will increase to €0.54 from €0.47 per ordinary share and to €0.56 from €0.49 per preference share. The previous year's dividend was adjusted to the share split.
In addition, shareholders affirmed the actions and decisions taken by the Supervisory Board and the Management Board in 2007 with a majority of more than 99%.
At the Annual General Meeting, 72.06% of the ordinary share capital and 2.48% of the preference share capital were represented. Only ordinary shareholders were entitled to vote.
Fresenius Medical Care is the world's largest integrated provider of products and services for individuals undergoing dialysis because of chronic kidney failure, a condition that affects more than 1,600,000 individuals worldwide. Through its network of 2,297 dialysis clinics in North America, Europe, Latin America, Asia-Pacific and Africa, Fresenius Medical Care provides dialysis treatment to 177,059 patients around the globe. Fresenius Medical Care is also the world's leading provider of dialysis products such as hemodialysis machines, dialyzers and related disposable products. Fresenius Medical Care is listed on the Frankfurt Stock Exchange (FME, FME3) and the New York Stock Exchange (FMS, FMS/P).
For more information about Fresenius Medical Care visit the Company's website at www.fmc-ag.com.
This release contains forward-looking statements that are subject to various risks and uncertainties. Future results could differ materially from those described in these forward-looking statements due to certain factors, e.g. changes in business, economic and competitive conditions, regulatory reforms, results of clinical trials, foreign exchange rate fluctuations, uncertainties in litigation or investigative proceedings, and the availability of financing. Fresenius does not undertake any responsibility to update the forward-looking statements in this release.
Moody's has raised the corporate credit rating of Fresenius SE from Ba2 to Ba1. The ratings of the Senior Notes issued by the company's wholly-owned subsidiary Fresenius Finance B.V. also improved. The € 100 million Senior Notes due 2009 with a coupon of 7.5 %, the € 500 million Senior Notes due 2013 with a coupon of 5.0 % and the € 500 million Senior Notes due 2016 with a coupon of 5.5 % have been upgraded to Ba1 as well.
Moody's has also upgraded the corporate credit rating of Fresenius Medical Care AG & Co. KGaA from Ba2 to Ba1 and has raised the ratings of its debt.
A stable outlook has been assigned to all ratings.
Moody's has based the upgrade on the continuous improvements in the operating performance of both companies, evidenced by increased levels of profitability and cash flow generation driving a reduction of financial leverage.
Fresenius is a health care group with international operations, providing products and services for dialysis, hospital and outpatient medical care. In 2007, group sales were approx. € 11.4 billion. On March 31, 2008 the Fresenius Group had 116,203 employees worldwide.
For more information visit the Company's website at www.fresenius.com.
This release contains forward-looking statements that are subject to certain risks and uncertainties. Future results could differ materially from those described in these forward-looking statements due to various factors, e.g., changes in the business, economic and competitive environment, regulatory reforms, results of clinical trials, foreign exchange rate fluctuations, uncertainties in litigation or investigative proceedings, and the availability of financing. Fresenius does not undertake any responsibility to update the forward-looking statements in this release.
Board of Management: Dr. Ulf M. Schneider (President and CEO), Rainer Baule, Dr. Francesco De Meo, Dr. Jürgen Götz, Dr. Ben Lipps, Stephan Sturm, Dr. Ernst Wastler
Supervisory Board: Dr. Gerd Krick (Chairman)
Registered Office: Bad Homburg, Germany/Commercial Register No. HRB 10660
The ratings of Fresenius Medical Care debt instruments have also been advanced. The Senior Secured Debt under the $4.6 billion Credit Agreement of Fresenius Medical Care AG & Co. KGaA received a Baa3 Investment Grade Rating. The rating of the Unsecured $500 million Senior Notes 2007-2017 issued by FMC Finance III S.A. with a coupon of 6 7/8% has been improved from Ba3 to Ba2. The ratings of the $225 million Senior Subordinated Notes of Fresenius Medical Care Capital Trust IV with a coupon of 7 7/8% and the €300 million Senior Subordinated Notes of Fresenius Medical Care Capital Trust V with a coupon of 7 3/8% were raised from B1 to Ba3.
A stable outlook has been assigned to all ratings.
Lawrence A. Rosen, Chief Financial Officer, commented: "With this upgrade Fresenius Medical Care is back to the rating level before the 100% debt-financed acquisition of Renal Care Group took place two years ago. The upgrade is the result of the Company's financial discipline and our proven ability to reduce leverage significantly over time. It also reflects our position as the leader in the dialysis industry, our good cost control and operating efficiency improvements as well as our continued strong cash flow generation."
Fresenius Medical Care is the world's largest integrated provider of products and services for individuals undergoing dialysis because of chronic kidney failure, a condition that affects more than 1,600,000 individuals worldwide. Through its network of 2,297 dialysis clinics in North America, Europe, Latin America, Asia-Pacific and Africa, Fresenius Medical Care provides dialysis treatment to 177,059 patients around the globe. Fresenius Medical Care is also the world's leading provider of dialysis products such as hemodialysis machines, dialyzers and related disposable products. Fresenius Medical Care is listed on the Frankfurt Stock Exchange (FME, FME3) and the New York Stock Exchange (FMS, FMS/P).
For more information about Fresenius Medical Care visit the Company's website at www.fmc-ag.com.
This release contains forward-looking statements that are subject to various risks and uncertainties. Actual results could differ materially from those described in these forward-looking statements due to certain factors, including changes in business, economic and competitive conditions, regulatory reforms, foreign exchange rate fluctuations, uncertainties in litigation or investigative proceedings, and the availability of financing. These and other risks and uncertainties are detailed in Fresenius Medical Care AG & Co. KGaA's reports filed with the U.S. Securities and Exchange Commission. Fresenius Medical Care AG & Co. KGaA does not undertake any responsibility to update the forward-looking statements in this release.
Following the Company's record financial results in 2007, Fresenius continues to expect strong sales and earnings growth in 2008. At the Annual General Meeting in Frankfurt Fresenius' Chairman of the Management Board Dr. Ulf M. Schneider confirmed the positive outlook for the full year 2008. Fresenius expects Group sales to increase by 8 to 10% in constant currency. Net income is expected to increase by 10 to 15% in constant currency. "Our growth prospects and strategic opportunities are excellent. The strong demand for high-quality healthcare drives sustainable organic growth. Selective acquisitions will further support our international business expansion. We are well positioned to take advantage of the opportunities in the fast-growing healthcare market," Schneider said.
During the Annual General Meeting, Fresenius shareholders approved the 15th consecutive dividend increase with a majority of more than 99%. Holders of ordinary shares will receive € 0.66 per share (2006: € 0.57) and holders of preference shares will receive € 0.67 (2006: € 0.58). This is an increase of about 15%. The total dividend distribution is € 103.2 million (2006: € 88.8 million).
Shareholders also elected a new supervisory board. Strategy Consultant Prof. Dr. h.c. Roland Berger and Klaus-Peter Müller, Chairman of the Supervisory Board of Commerzbank AG, will join the twelve-member board.
89.72 percent of the ordinary share capital and 59.17 percent of the preference share capital was represented at Fresenius SE's Annual General Meeting. A broad majority of more than 99 percent approved the actions of the Management and Supervisory Boards in 2007.
Fresenius is a health care group with international operations, providing products and services for dialysis, hospital and outpatient medical care. In 2007, group sales were approx. € 11.4 billion. On March 31, 2008 the Fresenius Group had 116,203 employees worldwide.
For more information visit the Company's website at www.fresenius.com.
This release contains forward-looking statements that are subject to certain risks and uncertainties. Future results could differ materially from those described in these forward-looking statements due to various factors, e.g., changes in the business, economic and competitive environment, regulatory reforms, results of clinical trials, foreign exchange rate fluctuations, uncertainties in litigation or investigative proceedings, and the availability of financing. Fresenius does not undertake any responsibility to update the forward-looking statements in this release.
Board of Management: Dr. Ulf M. Schneider (President and CEO), Rainer Baule, Dr. Francesco De Meo, Dr. Jürgen Götz, Dr. Ben Lipps, Stephan Sturm, Dr. Ernst Wastler
Supervisory Board: Dr. Gerd Krick (Chairman)
Registered Office: Bad Homburg, Germany/Commercial Register No. HRB 10660
The path from a brilliant idea to a market-ready product is often long and arduous. Fresenius wants to help inventors along this path to support the spread of innovative concepts from all areas of medicine. The health care group is now announcing the 10th Fresenius Inventors' Fair. Creative types can present their ideas from November 19 to 21, 2008 as part of MEDICA, the world's largest medical trade fair in Dusseldorf. The Inventors' Fair offers an international forum for innovators to find professional partners from the private sector as well as potential investors for the further development and marketing of their ideas. On the first day of the trade fair, the three best inventions are awarded the Fresenius Inventors' Prize, worth 10,000 Euro in total. Applications are currently being accepted.
There are plenty of new developments in diagnostics and therapies as well as medical devices, care and rehabilitation. But for the most possible patients to benefit from these innovations, inventors often need a healthy dose of perseverance and additional expertise. The Fresenius Inventors' Fair offers innovators an opportunity to present their concepts to experts and the media, and to secure specialist and financial support from interested companies. This increases the chance that their idea could enter mass production and benefit from professional marketing.
An independent jury of medical and business specialists will select the three best innovations. Emphasis is placed on the potential benefits and novelty of an idea. "Feasibility is also a key trait of winning innovations," explains Martin Hepper, doctor and member of the 2006 Inventors' Prize jury. One such successful entry came from Orthopedic doctor Michael Arnold: he developed two orthopedic surgical instruments that allow a relatively gentle removal of worn prostheses, regardless of the original model or manufacturer.
Doctors, natural scientists, engineers as well as hospital and care professionals may now submit their ideas. Fresenius will provide select applicants with exhibition space and stands free of charge at the Inventors' Fair. The health care group has again invested in the professional design of the stands. This should allow exhibitors to showcase their innovations while stimulating interesting discussions with specialists and media representatives. Twenty exhibitors took part in the 2006 Inventors' Fair and numerous specialists from a variety of medical specialists entered the competition.
The deadline for applications is October 2, 2008. The Inventors' Fair will be in Hall 8b during MEDICA. The trade fair in Dusseldorf runs from November 19 to 21, 2008 and is open from 10 a.m. to 6:30 p.m. Additional information on the Inventors' Fair may be found at http://www.fresenius-erfindermesse.de/, and on MEDICA at http://www.medica.de. Contact: Fresenius SE, codeword: "Inventors' Fair", 61346 Bad Homburg, Germany, Fax: 0049-61 72-6 08 22 94, E-Mail: daniela.hegemann@fresenius.com.
Fresenius is a health care group with international operations, providing products and services for dialysis, hospital and outpatient medical care. In 2007, group sales were approx. € 11.4 billion. On March 31, 2008 the Fresenius Group had 116,203 employees worldwide.
For more information visit the Company's website at www.fresenius.com.
This release contains forward-looking statements that are subject to various risks and uncertainties. Future results could differ materially from those described in these forward-looking statements due to certain factors, e.g. changes in business, economic and competitive conditions, regulatory reforms, results of clinical trials, foreign exchange rate fluctuations, uncertainties in litigation or investigative proceedings, and the availability of financing. Fresenius does not undertake any responsibility to update the forward-looking statements in this release.
Board of Management: Dr. Ulf M. Schneider (President and CEO), Rainer Baule, Dr. Francesco De Meo, Dr. Jürgen Götz, Dr. Ben Lipps, Stephan Sturm, Dr. Ernst Wastler
Supervisory Board: Dr. Gerd Krick (Chairman)
Registered Office: Bad Homburg, Germany/Commercial Register No. HRB 10660
Fresenius Kabi, a business segment of the health care group Fresenius, has signed definitive agreements to acquire APP Pharmaceuticals, Inc. APP is a leading manufacturer of intravenously administered generic drugs (I.V. generics) in North America. The company is listed on the NASDAQ Stock Market.
APP shareholders will receive a Cash Purchase Price of US$ 23.00 per share and a registered and tradeable Contingent Value Right (CVR) that could deliver up to US$ 6.00 per share, payable in 2011, if APP exceeds a cumulative adjusted EBITDA target for 2008 to 2010.
Based on the Cash Purchase Price, the transaction values the fully diluted equity capital of APP at approximately US$ 3.7 billion. Fresenius expects the acquisition to be neutral to EPS in the first year and clearly accretive from the second year onwards.
The acquisition is an important step in Fresenius Kabi's growth strategy. Through the acquisition of APP, Fresenius Kabi enters the U.S. pharmaceuticals market and achieves a leading position in the global I.V. generics market. This North American platform provides further attractive growth opportunities for Fresenius Kabi's existing product portfolio.
APP focuses on I.V. generics for hospital use and distributes its products in the U.S. and Canada. The company employs around 1,400 people and has state-of-the-art production facilities in Illinois, New York and Puerto Rico as well as a subsidiary in Toronto, Canada.
With a portfolio of more than 100 products, comprising drugs for oncology, intensive care, anaesthesia, analgesia as well as drugs for the treatment of infections, APP holds an important position in the North American hospital market. The company has a strong drug registration pipeline covering all of its product segments.
In 2007, APP achieved sales of US$ 647 million and an adjusted EBITDA of US$ 253 million. The company's latest published guidance for 2008 forcasts sales in a range of US$ 730 million to US$ 750 million and an adjusted EBITDA in a range of US$ 285 million to US$ 300 million.
As part of this transaction APP will merge with a U.S. subsidiary of Fresenius Kabi. The definitive agreements include a written consent and voting agreement with Dr. Patrick Soon-Shiong, APP's founder and shareholder of over 80 % of the outstanding stock. The transaction has been approved by APP's Board of Directors.
Following the closing of the transaction, Patrick Soon-Shiong will serve as a non-executive director on the Board of Fresenius Kabi's U.S. Holding company. In this role, he will continue to contribute to the company's strategic development.
Dr. Ulf Mark Schneider, Chairman of the Management Board of Fresenius SE commented: "APP is a fast-growing, highly profitable company and a strong management team that has an excellent market position in the U.S. Our firm very much shares APP's dedication to quality and medical excellence for the benefit of patients. The acquisition provides significant growth opportunities for Fresenius Kabi. With the APP platform, Fresenius Kabi will be able to market its product range in the U.S. Fresenius Kabi's international marketing and sales network will allow us to sell APP's products globally. We welcome APP employees to our team and very much look forward to serving the North American healthcare community."
Patrick Soon-Shiong said: "We are proud to have consistently provided injectable pharmaceutical products of the highest quality to patients in the acute care setting over the past decade. In Fresenius we have found a partner with the same commitment to quality and dedication to patient care. The combined company will allow for the rapid globalization of APP's portfolio with the same high levels of quality and patient commitment for which we have become known, while at the same time providing a more comprehensive and complementary offering of injectable pharmaceuticals, devices and delivery systems to customers worldwide."
It is planned to finance the acquisition with a mix of debt and equity, targeted to minimize the impact on Fresenius SE's credit ratings. However, given Fresenius' rapid progress in de-levering since 2006, the largest portion of the financing will consist of debt instruments.
Financing commitments for the total amount have been received from Deutsche Bank, Credit Suisse and JP Morgan. Details of the financing plan will be published in the coming weeks. Deutsche Bank acts as Global Coordinator of the financing and as sole M&A advisor to Fresenius.
The transaction is subject to certain closing conditions, including regulatory approvals, and approvals under the Hart-Scott-Rodino Antitrust Improvements Act of 1976. Fresenius anticipates closing the transaction at the end of 2008 or beginning of 2009.
EPS: before one-time transaction-related depreciation charges and assuming a 2008 closing.
Adjusted EBITDA: EBITDA before one-time expenses and stock-option expenses, as published by APP in Form 8-K, March 10, 2008, as of Dec 31, 2007.
APP is a fully-integrated pharmaceutical company that develops, manufactures and markets injectable pharmaceutical products with a primary focus on the oncology, anti-infective, anesthetic/analgesic and critical care markets. The Company offers one of the most comprehensive product portfolios used in hospitals, long-term care facilities, alternate care sites and clinics within North America and manufactures a comprehensive range of dosage formulations
Fresenius is a health care group with international operations, providing products and services for dialysis, hospital and outpatient medical care. In 2007, group sales were approx. € 11.4 billion. On March 31, 2008 the Fresenius Group had 116,203 employees worldwide.
For more information visit the Company's website at www.fresenius.com.
THIS RELEASE IS FOR INFORMATION PURPOSES ONLY AND MAY NOT BE FURTHER DISTRIBUTED OR PASSED ON TO ANY OTHER PERSON OR PUBLISHED, IN WHOLE OR IN PART, FOR ANY PURPOSE.
This release does not constitute or form part of, and should not be construed as, an offer or invitation to subscribe for, underwrite or otherwise acquire, any securities of Fresenius SE ("Fresenius") or any present or future member of its group nor should it or any part of it form the basis of, or be relied on in connection with, any contract to purchase or subscribe for any securities of Fresenius or any member of its group or any commitment whatsoever. In particular, this release is not an offer of securities in the United States of America (including its territories and possessions), and securities of Fresenius SE may not be offered or sold in the United States of America absent registration under the Securities Act of 1933 (which Fresenius SE does not intend to effect) or pursuant to an exemption from registration.
This release contains forward-looking statements that are subject to various risks and uncertainties. Future results could differ materially from those described in these forward-looking statements due to certain factors, e.g. changes in business, economic and competitive conditions, regulatory reforms, results of clinical trials, foreign exchange rate fluctuations, uncertainties in litigation or investigative proceedings, and the availability of financing. This includes the risk that the transaction will not be consummated or on other terms. Fresenius does not undertake any responsibility to update the forward-looking statements in this release.
This document is directed at and/or for distribution in the U.K. only to (i) persons who have professional experience in matters relating to investments falling within article 19(5) of the Financial Services and Markets Act 2000 (Financial Promotion) Order 2005 (the "Order") or (ii) high net worth entities falling within article 49(2)(a) to (d) of the Order (all such persons being together referred to as "relevant persons"). This document is directed only at relevant persons. Other persons should not act or rely on this document or any of its contents.
The information contained herein is not for publication or distribution in Canada, Australia or Japan and does not constitute an offer of securities for sale in Canada, Australia or Japan.
Board of Management: Dr. Ulf M. Schneider (President and CEO), Rainer Baule, Dr. Francesco De Meo, Dr. Jürgen Götz, Dr. Ben Lipps, Stephan Sturm, Dr. Ernst Wastler
Supervisory Board: Dr. Gerd Krick (Chairman)
Registered Office: Bad Homburg, Germany/Commercial Register No. HRB 10660
Fresenius Medical Care AG & Co. KGaA, the world's largest provider of dialysis products and services, today announced that it signed exclusive license agreements for the intravenous (IV) Iron products Venofer® (iron sucrose) and Ferinject® (ferric carboxymaltose) to treat iron deficiency anemia experienced by dialysis patients. Venofer® is the leading IV Iron product worldwide.
Galenica Ltd., its subsidiary Vifor Pharma and Fresenius Medical Care are entering into a strategic joint venture for dialysis to market and distribute the two iron products Venofer® and Ferinject® in Europe, Middle East, Africa and Latin America. The agreement concerns all commercialization activities for these IV Iron products in the field of dialysis and is expected to become effective not later than January 1st, 2009.
Commercialization of these respective IV Iron products outside the field of dialysis will remain fully the responsibility of Vifor Pharma and its existing key partners.
The total market for IV Iron in Europe, Middle East, Africa and Latin America was greater than $120 million in 2007. After the first year of the agreement, Fresenius Medical Care expects yearly sales from both IV Iron products to be in excess of $50 million.
In North America, a second Agreement provides for an exclusive U.S. manufacturing and distribution sublicense for Venofer® with Luitpold Pharmaceuticals Inc (Luitpold). In addition, it includes a similar sublicense for the next generation of iron products for the U.S. and Canada known as Injectafer® (ferric carboxymaltose) injection, which is expected to enhance the treatment of anemia in the dialysis patient population through the application of innovative drug administration techniques. The transaction is subject to customary closing conditions including expiration of the applicable waiting period under the Hart-Scott-Rodino Antitrust Act. The closing of the transaction is anticipated in 2008.
Luitpold will continue to sell Venofer® for use in treating chronic kidney disease patients not yet on dialysis and in treating patients with acute renal failure in hospitals. Venofer® is currently the market leader in sales in the injectable iron market in the U.S. Currently, the total purchases of IV Iron in the U.S are approximately US$ 500 million. Venofer® has a U.S. market share of approximately 55%. Galenica Ltd., through its Vifor Pharma subsidiary, exclusively licenses the Venofer® and Injectafer® products to Luitpold and American Regent for the U.S. and Canada.
As part of the 10-year Agreement for North America, Luitpold will continue to contract manufacture the products for Fresenius Medical Care. Luitpold will continue to pay royalties to Vifor Pharma, based in St. Gallen, Switzerland, which supplies the active pharmaceutical ingredient to Luitpold.
Ben Lipps, Chief Executive Officer of Fresenius Medical Care, commented: "We are very pleased to have Galenica Ltd., Vifor Pharma, Luitpold Pharmaceuticals, Inc. and American Regent, Inc., as our partners dedicated to improving the treatment of iron deficiency anemia experienced by dialysis patients. It is an excellent opportunity for us to access a proven and effective IV Iron product portfolio. With these agreements we will also deploy our "Pharmatech" strategy, aimed to introduce new drug delivery systems to improve quality and safety of dialysis treatments."
Fresenius Medical Care is the world's largest integrated provider of products and services for individuals undergoing dialysis because of chronic kidney failure, a condition that affects more than 1,600,000 individuals worldwide. Through its network of 2,297 dialysis clinics in North America, Europe, Latin America, Asia-Pacific and Africa, Fresenius Medical Care provides dialysis treatment to 177,059 patients around the globe. Fresenius Medical Care is also the world's leading provider of dialysis products such as hemodialysis machines, dialyzers and related disposable products. Fresenius Medical Care is listed on the Frankfurt Stock Exchange (FME, FME3) and the New York Stock Exchange (FMS, FMS/P). For more information about Fresenius Medical Care visit the Company's website at www.fmc-ag.com.
Galenica is a diversified group active throughout the healthcare market which, among other things, develops, manufactures and commercialises pharmaceutical products, runs pharmacies, provides logistical and database services and sets up networks. The Galenica Group enjoys a leading position in all its business sectors – Pharma, Logistics, HealthCare Information and Retail. A large part of the Group's income is generated by international operations. Additional information on the Galenica Group can be found at www.galenica.com.
Luitpold Pharmaceuticals, Inc., headquartered in Shirley, NY, manufactures and distributes over 65 pharmaceutical products including Venofer® (iron sucrose injection, USP), the leading IV iron therapy in the U.S., through its human health subsidiary, American Regent, Inc. Luitpold Pharmaceuticals, Inc., a Daiichi-Sankyo Group company, also markets dental bone regeneration products and veterinary pharmaceuticals through its Osteohealth and Animal Health divisions. Daiichi Sankyo Company, Ltd., established in 2005 after the merger of two leading century-old Japanese pharmaceutical companies, is a global pharmaceutical innovator, continuously generating innovative drugs that enrich the quality of life for patients around the world. For more information about Luitpold see www.luitpold.com.
This release contains forward-looking statements that are subject to various risks and uncertainties. Future results could differ materially from those described in these forward-looking statements due to certain factors, e.g. changes in business, economic and competitive conditions, regulatory reforms, results of clinical trials, foreign exchange rate fluctuations, uncertainties in litigation or investigative proceedings, and the availability of financing. Fresenius does not undertake any responsibility to update the forward-looking statements in this release.



