- Sales: € 10.4 billion, +19 % at actual rates, +15 % in constant currency
- EBIT: € 1.5 billion, +24 % at actual rates, +19 % in constant currency
- Adjusted net income*: € 368 million, +14 % at actual rates, +12 % in constant currency
- Ongoing sales and earnings growth across all business segments
- Fresenius Medical Care improves guidance
- Fresenius Kabi confirms guidance - number of product approvals at APP Pharmaceuticals increased
- Fresenius Helios raises earnings guidance
- Fresenius Vamed confirms guidance
- Excellent cash flow results in significantly reduced leverage
Group outlook for 2009 fully confirmed
Based on the Group's strong financial results, Fresenius fully confirms its positive outlook for 2009. Group sales are expected to grow by more than 10 % in constant currency.
Organic growth is projected to be in a 6 to 8 % range. Adjusted net income* is expected to increase by approximately 10 % in constant currency.
Fresenius plans to invest € 700 to 750 million in property, plant and equipment.
* Net income attributable to Fresenius SE; adjusted for the effects of mark-to-market accounting of the Mandatory Exchangeable Bonds (MEB) and the Contingent Value Rights (CVR) relating to the acquisition of APP Pharmaceuticals. Both are non-cash charges.
Strong sales growth across all business segments continued
Group sales increased by 15 % in constant currency and by 19 % at actual rates to € 10,429 million (Q1-3/2008: € 8,761 million). Organic sales growth was 8 % for the first three quarters and increased to 9 % in the third quarter. Acquisitions contributed a further 7 %. Currency translation had a positive impact of 4 %. This is mainly attributable to the average US dollar rate improving 10 % against the euro in the first three quarters of 2009.
Sales growth in the business segments was as follows:


In Europe sales grew by 11 % in constant currency with organic sales growth contributing 8 %. In North America sales grew by 20 % in constant currency, mainly due to the consolidation of APP Pharmaceuticals from September 2008. Strong organic growth rates were achieved in the emerging markets, reaching 13 % in Asia-Pacific and 12 % in Latin America.


Continued strong earnings growth
Group EBITDA increased by 19 % in constant currency and by 24 % at actual rates to € 1,911 million (Q1-3/2008 adjusted: € 1,546 million). Group operating income (EBIT) grew by 19 % in constant currency and by 24 % at actual rates to € 1,496 million (Q1-3/2008 adjusted: € 1,209 million). The Group's EBIT margin increased to 14.3 % (Q1-3/2008 adjusted: 13.8 %).
Group net interest was € -439 million (Q1-3/2008: € -271 million). Lower average interest rates on liabilities of Fresenius Medical Care were more than offset by incremental debt relating to the acquisitions of APP Pharmaceuticals and Dabur Pharma and currency translation effects.
The other financial result was € -30 million and includes valuation changes of the fair redemption value of the Mandatory Exchangeable Bonds (MEB) of € -3 million and the Contingent Value Rights (CVR) of € -27 million. Both are non-cash charges.
The adjusted Group tax rate* was 30.8 % (Q1-3/2008 adjusted: 34.2 %). This decrease was largely driven by the revaluation of a tax claim at Fresenius Medical Care in Q2 2009.
Noncontrolling interest increased to € 363 million (Q1-3/2008: € 293 million), of which 94 % was attributable to the noncontrolling interest in Fresenius Medical Care.
Adjusted net income** grew by 12 % in constant currency and by 14 % at actual rates to € 368 million (Q1-3/2008 adjusted: € 324 million). Adjusted earnings per ordinary share increased to € 2.28 and adjusted earnings per preference share increased to € 2.29 (Q1-3/2008 adjusted: ordinary share € 2.06, preference share € 2.07). This represents an increase of 11 % at actual rates and 9 % in constant currency for both share classes.
The Group's US GAAP financial results as of September 30, 2009 and as of September 30, 2008, include the effects of mark-to-market accounting of the Mandatory Exchangeable Bonds (MEB) and the Contingent Value Rights (CVR) relating to the acquisition of APP Pharmaceuticals. Those special items are recognized in the financial result of the "Corporate/Other" segment. Adjusted earnings represent the Group's business operations in the reporting period. In the previous year's period, the Group's US GAAP financial statements include in addition several special items relating to the acquisition of APP Pharmaceuticals. Please see page 14 of this Investor News for the reconciliation to earnings according to US GAAP.
Net income*** (including special items) was € 339 million or € 2.10 per ordinary share and € 2.11 per preference share.
* Adjusted for the effects of mark-to-market accounting of the Mandatory Exchangeable Bonds (MEB) and the Contingent Value Rights (CVR) relating to the acquisition of APP Pharmaceuticals.
** Net income attributable to Fresenius SE; adjusted for the effects of mark-to-market accounting of the Mandatory Exchangeable Bonds (MEB) and the Contingent Value Rights (CVR) relating to the acquisition of APP Pharmaceuticals. Both are non-cash charges.
*** Net income attributable to Fresenius SE.
Continued investments in growth
Fresenius Group spent € 442 million for property, plant and equipment (Q1-3/2008: € 502 million). Acquisition spending was € 186 million (Q1-3/2008: € 3,760 million, mainly due to the acquisition of APP Pharmaceuticals).
Strong cash flow achieved
Operating cash flow increased by 52 % to € 1,120 million (Q1-3/2008: € 736 million), driven by strong earnings growth and tight working capital management. Net capital expenditure was € 446 million (Q1-3/2008: € 496 million). Cash flow before acquisitions and dividends nearly tripled to € 674 million (Q1-3/2008: € 240 million).
Balance sheet
Fresenius Group's total assets were € 20,632 million (December 31, 2008: € 20,544 million). In constant currency, total assets increased by 3 %. Current assets increased by 6 % to € 5,408 million (December 31, 2008: € 5,078 million). Non-current assets decreased by 2 % to € 15,224 million (December 31, 2008: € 15,466 million).
Total shareholders' equity increased by 4 % to € 7,237 million (December 31, 2008: € 6,943 million). The equity ratio (including noncontrolling interest) improved to 35.1 % (December 31, 2008: 33.8 %).
Group debt decreased by 4 % to € 8,476 million (December 31, 2008: € 8,787 million).
As of September 30, 2009, the net debt/EBITDA ratio was 3.1 and significantly improved from 3.6 at December 31, 2008 (pro forma the acquisition of APP Pharmaceuticals and excluding special items).
Number of employees increased
As of September 30, 2009, Fresenius increased the number of its employees by 6 % to 129,218 (December 31, 2008: 122,217).
Fresenius Biotech
Fresenius Biotech develops innovative therapies with trifunctional antibodies for the treatment of cancer. In the field of polyclonal antibodies, Fresenius Biotech has successfully marketed ATG-Fresenius S for many years. ATG-Fresenius S is an immunosuppressive agent used to prevent and treat graft rejection following organ transplantation.
On April 22, 2009, the European Commission granted Fresenius Biotech the approval for Removab (catumaxomab) for the treatment of malignant ascites. Removab was launched in Germany in May 2009. Market launch is under way in other European countries. As of September 30, 2009, Fresenius Biotech achieved Removab sales of more than € 1 million.
Fresenius Biotech's EBIT was € -32 million in the first three quarters of 2009 (Q1-3/2008: € -32 million). For 2009, Fresenius Biotech expects its EBIT to reach € -40 million to € -45 million.
The Business Segments
Fresenius Medical Care
Fresenius Medical Care is the world's leading provider of services and products for patients with chronic kidney failure. As of September 30, 2009, Fresenius Medical Care was treating 192,804 patients in 2,509 dialysis clinics.


- Continued strong organic sales growth of 8 %
- Q3 EBIT margin improved to 15.6 (Q2 2009: 15.1 %)
- Improved guidance for 2009
Fresenius Medical Care achieved sales growth of 4 % to US$ 8,212 million (Q1-3/2008: US$ 7,890 million). Organic growth was 8 %. Currency translation effects had a negative impact of 5 %.
Dialysis services revenue grew by 6 % to US$ 6,124 million (Q1-3/2008: US$ 5,753 million), an increase of 9 % in constant currency. Sales of dialysis products were US$ 2,088 million (Q1-3/2008: US$ 2,137 million). In constant currency, dialysis products sales increased by 8 %.
In North America sales increased by 9 % to US$ 5,600 million (Q1-3/2008: US$ 5,153 million). Dialysis services revenue grew by 8 % to US$ 4,994 million. Average revenue per treatment for the U.S. clinics increased to US$ 348 in Q3 2009 compared to US$ 333 for Q3 2008 and 344 US$ for Q2 2009. This development was mainly based on reimbursement increases and increased utilization of pharmaceuticals. Sales outside North America ("International" segment) were US$ 2,612 million (Q1-3/2008: US$ 2,737 million). In constant currency, sales growth was 9 %.
EBIT increased by 2 % to US$ 1,265 million (Q1-3/2008: US$ 1,240 million), resulting in an EBIT margin of 15.4 % (Q1-3/2008: 15.7 %). This development was mainly due to higher personnel expenses, price increases for pharmaceuticals as well as the impact of the launch of a generic version of PhosLo® in the U.S. market. These effects were partially offset by a strong performance of the dialysis product business, increased commercial payor revenue as well as the effect of cost control measures.
Net income* increased by 7 % to US$ 645 million (Q1-3/2008: US$ 603 million).
For the full year of 2009, Fresenius Medical Care now expects to achieve revenue of around US$ 11.2 billion (previously US$ 11.1 billion), an increase of around 8 % in constant currency.
Net income* is now expected to be between US$ 865 million and US$ 890 million in 2009. Previously, the company expected the net income to be in the range of US$ 850 million and US$ 890 million for the full year 2009.
* Net income attributable to Fresenius Medical Care AG & Co. KGaA
For further information, please see Fresenius Medical Care's Press Release at www.fmc-ag.com.
Fresenius Kabi
Fresenius Kabi offers infusion therapies, intravenously administered generic drugs and clinical nutrition for seriously and chronically ill patients in the hospital and out-patient environments. The company is also a leading provider of medical devices and transfusion technology products.


- Organic sales growth accelerated to 8 %
- EBIT Margin increased to 19.4 %
- Outlook 2009 confirmed
Fresenius Kabi increased sales by 31 % to € 2,274 million (Q1-3/2008: € 1,734 million). Organic sales growth was 8 % in the first three quarters and increased to 9 % in the third quarter. Net acquisitions contributed 25 % to sales. Currency translation had a net negative impact of 2 %. This was mainly due to the depreciation of currencies in Great Britain and Brazil against the euro, whereas positive translation effects resulted from the strengthening of the Chinese yuan.
In Europe, sales reached € 1,159 million, driven by 5 % organic growth. In North America, sales increased to € 527 million (Q1-3/2008: € 134 million) due to the acquisition of APP Pharmaceuticals. In the Asia-Pacific region Fresenius Kabi achieved sales of € 361 million. Organic sales growth accelerated to 15 %. Sales in Latin America and Africa increased to € 227 million, driven by 16 % organic growth.
EBIT grew by 52 % to € 441 million (Q1-3/2008: € 290 million). EBIT includes a € 20 million non-cash charge related to the amortization of APP Pharmaceuticals' intangible assets. The EBIT margin increased to 19.4 % (Q1-3/2008: 16.7 %). Net interest grew to € 231 million (Q1-3/2008: € 64 million) due to the acquisition financing. Net income* was € 136 million (Q1-3/2008: € 149 million).
Sales at APP Pharmaceuticals increased by 16 % to US$ 632 million. APP Pharmaceuticals achieved significant sales growth in the product portfolio excluding Heparin in Q3, leading to a sales growth of 4 % in the first three quarters 2009. Adjusted EBITDA** grew by 20 % to US$ 260 million. EBIT was US$ 198 million. EBIT includes a US$ 27 million non-cash charge related to the amortization of intangible assets. The EBIT margin improved to 31.3 %. The number of product approvals from the FDA (Food and Drug Administration) has currently increased to seven, following only one approval in the first half of 2009.
Operating cash flow of Fresenius Kabi more than doubled to € 311 million (Q1-3/2008: € 144 million). This was primarily achieved through a tight working capital management. Given only moderate growth in capital expenditures, cash flow before acquisitions and dividends more than tripled to € 224 million (Q1-3/2008: € 69 million).
Fresenius Kabi confirms its outlook for 2009: the company targets sales growth in constant currency of 25 to 30 %. Further, Fresenius Kabi forecasts an EBIT margin in the range of 19.5 to 20.5 %. Currency translation effects may impact Fresenius Kabi's margin as APP Pharmaceuticals provides a significant earnings contribution from the US$ area. This guidance is based on the US$/€ exchange rate from the beginning of 2009.
Special items relating to the acquisition of APP Pharmaceuticals are included in the segment "Corporate/Other".
* Net income attributable to Fresenius Kabi AG
** Non-GAAP financial measures - Adjusted EBITDA is a defined term in the indenture governing the Contingent Value Rights (CVRs), however it is not a recognized term under GAAP.
Fresenius Helios
Fresenius Helios is one of the largest private hospital operators in Germany. The HELIOS Kliniken Group owns 62 hospitals, including five maximum care hospitals in Berlin-Buch, Erfurt, Krefeld, Schwerin and Wuppertal. HELIOS treats about 600,000 in-patients per year at its clinics and operates a total of more than 18,000 beds.


- Organic sales growth accelerated to 6 %
- Excellent earnings development at the established clinics
- Sales outlook 2009 confirmed, EBIT guidance raised
Fresenius Helios increased sales by 13 % to € 1,768 million (Q1-3/2008: € 1,568 million). Strong organic growth of 6 % was again driven by a significant increase in admissions. Net acquisitions contributed 7 % to overall sales growth.
EBIT grew by 20 % to € 152 million (Q1-3/2008: € 127 million) due to the excellent business operations of the established clinics. The EBIT margin increased to 8.6 % (Q1-3/2008: 8.1 %). Net income* improved by 39 % to € 82 million (Q1-3/2008: € 59 million).
At HELIOS' established clinics, sales rose by 6 % to € 1,646 million. EBIT improved by 22 % to € 154 million. The EBIT margin increased to 9.4 % (Q1-3/2008: 8.1 %). The acquired clinics (consolidation <1 year) achieved sales of € 122 million and € -2 million EBIT.
Fresenius Helios confirms its sales outlook for 2009 and expects to achieve sales of more than € 2.3 billion. EBIT is now projected to reach more than € 200 million. The previous guidance was € 190 to 200 million.
* Net income attributable to HELIOS Kliniken GmbH
Fresenius Vamed
Fresenius Vamed offers engineering and services for hospitals and other health care facilities.


- Sales increased by 36 %
- Order backlog at new all-time-high, strong order intake achieved in Q3
- Outlook 2009 fully confirmed
Fresenius Vamed achieved excellent sales growth of 36 % to € 393 million (Q1-3/2008: € 290 million). Organic sales growth was 29 %. The clinics in the Czech Republic acquired from Fresenius Helios contributed 7 %. Sales in the project business rose by 46 % to € 244 million (Q1-3/2008: € 167 million). Sales in the service business increased by 21 % to € 149 million (Q1-3/2008: € 123 million).
EBIT grew by 7 % to € 15 million (Q1-3/2008: € 14 million). Significant sales growth driven by a strong project business diluted the EBIT margin to 3.8 % (Q1-3/2008: 4.8 %). Net income* of € 13 million was € 1 million below previous year's level due to a decrease in interest income as a result of lower interest rates.
The excellent development of order intake and order backlog continued: Fresenius Vamed increased the order intake by 29 % to € 313 million (Q1-3/2008: € 242 million). In Q3 2009, Fresenius Vamed more than doubled its order intake to € 157 million (Q3 2008: € 72 million). Fresenius Vamed was awarded a >€ 80 million order for the turnkey construction of a general hospital in Gabon. The project is scheduled to start in the fourth quarter of 2009. The construction work will take approximately two years.
The order backlog reached the new all-time-high of € 640 million (December 31, 2008: € 571 million).
Fresenius Vamed fully confirms its outlook for 2009 and expects to grow both sales and EBIT by approximately 10 %.
* Net income attributable to VAMED AG
Analyst Conference
As part of the publication of the results of the first three quarters of 2009 a conference call will be held on November 3, 2009 at 2.00 p.m. CET (8.00 a.m. EST). You are cordially invited to follow the conference call in a live broadcast over the Internet at www.fresenius.com see Investor Relations / Presentations. Following the call, a recording will be available.
Fresenius is a health care group with international operations, providing products and services for dialysis, hospital and outpatient medical care. In 2008, group sales were approx. € 12.3 billion. On September 30, 2009 the Fresenius Group had 129,218 employees worldwide.
For more information visit the Company's website at www.fresenius.com.
This release contains forward-looking statements that are subject to various risks and uncertainties. Future results could differ materially from those described in these forward-looking statements due to certain factors, e.g. changes in business, economic and competitive conditions, regulatory reforms, results of clinical trials, foreign exchange rate fluctuations, uncertainties in litigation or investigative proceedings, and the availability of financing. Fresenius does not undertake any responsibility to update the forward-looking statements in this release.
Board of Management: Dr. Ulf M. Schneider (President and CEO), Rainer Baule, Dr. Francesco De Meo, Dr. Jürgen Götz, Dr. Ben Lipps, Stephan Sturm, Dr. Ernst Wastler
Supervisory Board: Dr. Gerd Krick (Chairman)
Registered Office: Bad Homburg, Germany/Commercial Register No. HRB 10660
Fresenius Group in Figures
- Consolidated statement of income (US GAAP, unaudited)
- Consolidated statement of financial position (US GAAP, unaudited)
- Consolidated statement of cash flows (US GAAP, unaudited)
- Segment reporting by business segment H1 (US GAAP, unaudited)
- Segment reporting by business segment Q2 (US GAAP, unaudited)
see PDF-file
Fresenius Medical Care, the world's largest provider of dialysis services and products, today announced a transition to a new management board structure, drawing entirely on internal management strength. The Chairman's contract has been extended and a Deputy Chairman of the Fresenius Medical Care Management Board has been appointed which defines a successful leadership succession path. The Company has also appointed a new Chief Financial Officer. A new Fresenius Medical Care Management Board position for Global Manufacturing Operations has been established to provide stronger manufacturing coordination on a global basis. This will allow the Regional Chief Executive Officers more opportunity to expand the Renal Therapy and Service Business.
The contract of the Chairman of the Management Board and Chief Executive Officer Dr. Ben J. Lipps has been extended until December 31, 2012.
Effective January 1, 2010, Rice Powell has been promoted to be Deputy Chairman of the Fresenius Medical Care Management Board and Chief Executive Officer of Fresenius Medical Care North America. Rice has been with the Company since 1997, and has been the Co-CEO of Fresenius Medical Care North America and CEO of the Renal Therapies Group. Rice has been a member of the Fresenius Medical Care Management Board since 2004 and has more than 30 years of experience in the health care industry.
Mats Wahlstrom, Co-CEO of Fresenius Medical Care North America and a member of the Fresenius Medical Care Management Board, has informed the Company of his intention to reduce his full-time responsibilities with the Company effective during January 2010. He has been with Fresenius Medical Care since 2002 and was a member of the Fresenius Medical Care Management Board since 2004. Mats will step back from his responsibilities as a member of the Management Board but has accepted to stay on the Board of Directors of Fresenius Medical Care North America and continue as a senior advisor to the Chairman of the Fresenius Medical Care Management Board for a period of five years. In that capacity Mats will support the Company's focus on new therapies and services, global services expansion and leadership development.
Michael Brosnan has accepted the position as the Chief Financial Officer of Fresenius Medical Care effective January 1, 2010, and in this capacity will join the Fresenius Medical Care Management Board. For the past seven years, he has served as Chief Financial Officer and member of the Board of Directors of Fresenius Medical Care North America. Mike joined the Company in 1998 as Vice President of Finance and Administration for Spectra Renal Management, the Company's laboratory services organization. Since then, Mike has held several executive positions in North America. Prior to joining Fresenius Medical Care, Mike held senior financial positions at Polaroid Corporation and was an audit partner at KPMG.
Effective January 1, 2010, Kent Wanzek will be named to a new position on the Fresenius Medical Care Management Board with responsibility for Global Products Manufacturing Operations. Kent has been the President for Operations of the Renal Therapies Group at Fresenius Medical Care North America since 2006. Prior to joining the Company in 2003, Kent had several senior executive positions including Philips Medical Systems and Baxter Healthcare Corporation.
Dr. Emanuele Gatti, whose contract has been extended, will assume the responsibility as Chief Strategist for Fresenius Medical Care in addition to his capacity as a member of the Management Board and Chief Executive Officer for Europe, Middle East, Africa and Latin America. Under his successful leadership Fresenius Medical Care has grown to serve 50,000 patients, has become the leading manufacturer of dialysis products in this region and has set a new standard for quality and innovation.
Roberto Fustė will continue his position as Chief Executive Officer of Fresenius Medical Care Asia Pacific, which will ensure further successful leadership. During the last eight years and under the leadership of Roberto, Asia Pacific has grown from annual revenue of $86 million in 1997 to over $650 million annualized revenue today. Fresenius Medical Care Asia Pacific is projected to achieve annual revenue of over $1 billion by 2012.
The area of law, compliance and intellectual property will continue to benefit from Dr. Rainer Runte's leadership whose contract also had been renewed. Rainer will additionally become responsible for human resources in Germany (Labor Relations Director) and oversee the implementation of the Company's business development initiatives. Rainer joined Fresenius Medical Care in 1990 and was appointed to the Fresenius Medical Care Management Board in 2002.
Ben Lipps, Chief Executive Officer of Fresenius Medical Care and Chairman of the Management Board, said: "We are pleased with the decision to appoint Rice Powell Deputy Chairman of the Fresenius Medical Care Management Board and to appoint Michael Brosnan Chief Financial Officer as well as the creation of a new Board position for Global Product Manufacturing under Kent Wanzek's leadership. Rice Powell's successful record and his extensive management experience within Fresenius Medical Care and the health care field qualify him superbly for the Deputy Chairman position and I look forward to working with him closely in this role. Michael Brosnan, with his knowledge of Fresenius Medical Care and his proven financial experience, is an excellent addition to the Management Board.
We are deeply grateful to Mats Wahlstrom for his leadership during a period of rapid growth and consolidation in North America. During Mats tenure, the number of patients treated in North America grew from approximately 79,000 patients in 2002 to nearly 131,000 patients in 2009 with significant improvement in quality and financial performance. We are particularly proud of his leadership in the seamless integration of Renal Care Group and his development of a very strong management team. I personally would like to thank Mats for his exceptional contribution over the last years and I am pleased to be able to continue to rely on his judgment and guidance as we move forward.
These changes in the Fresenius Medical Care Management Board will serve the Company well as it faces future challenges and opportunities. The fact that all appointments have been filled internally reflects the strength and depth of our management team and will ensure a smooth transition going forward."
Ulf Mark Schneider, Chairman of the Supervisory Board of Fresenius Medical Care Management AG, said: "The dialysis industry is facing significant change with major growth opportunities in international markets and the introduction of the bundled reimbursement system in the U.S. In this context, we are very pleased that Ben Lipps will continue to lead this company as Chairman of the Management Board. With his four decades of dialysis industry experience and his outstanding track record in building our company he will provide proven leadership and stability in this important period.
At the same time, we are pleased to elevate Rice Powell to the new position of Deputy Chairman of the Management Board. Building on his significant accomplishments in our North American organization this role will prepare Rice for succeeding Ben in leading our company. We are very confident that Ben and Rice, together with the newly structured Management Board, will continue to successfully develop the company."
New Management Board Fresenius Medical Care
Dr. Ben J. Lipps, 69
Chief Executive Officer (CEO) and Chairman of the Management Board
Rice Powell, 54
CEO for North America and Deputy Chairman of the Management Board
Mike Brosnan, 54
Chief Financial Officer (CFO)
Roberto Fusté, 56
Chief Executive Officer for Asia-Pacific
Dr. Emanuele Gatti, 54
Chief Executive Officer for Europe, Middle East, Africa, Latin America and Global Chief Strategist
Dr. Rainer Runte, 50
Member of the Management Board responsible for Law, Compliance & Corporate Governance and Intellectual Property, Labor Relations Director for Germany
Kent Wanzek, 50
Member of the Management Board responsible for Global Production
Fresenius Medical Care is the world's largest integrated provider of products and services for individuals undergoing dialysis because of chronic kidney failure, a condition that affects more than 1,770,000 individuals worldwide. Through its network of 2,509 dialysis clinics in North America, Europe, Latin America, Asia-Pacific and Africa, Fresenius Medical Care provides dialysis treatment to 192,804 patients around the globe. Fresenius Medical Care is also the world's leading provider of dialysis products such as hemodialysis machines, dialyzers and related disposable products. Fresenius Medical Care is listed on the Frankfurt Stock Exchange (FME, FME3) and the New York Stock Exchange (FMS, FMS/P).
For more information about Fresenius Medical Care visit the Company's website at www.fmc-ag.com.
This release contains forward-looking statements that are subject to various risks and uncertainties. Actual results could differ materially from those described in these forward-looking statements due to certain factors, including changes in business, economic and competitive conditions, regulatory reforms, foreign exchange rate fluctuations, uncertainties in litigation or investigative proceedings, and the availability of financing. These and other risks and uncertainties are detailed in Fresenius Medical Care AG & Co. KGaA's reports filed with the U.S. Securities and Exchange Commission. Fresenius Medical Care AG & Co. KGaA does not undertake any responsibility to update the forward-looking statements in this release.
Fresenius Medical Care AG & Co. KGaA ("the Company") today announced that it has been selected by the National Health System (NHS) in the United Kingdom to manage dialysis care for 12 renal units across the North of England.
These clinics will provide dialysis treatments for more than 400 patients for approximately 7 years in North East and South Yorkshire and Lincolnshire. Patients will remain under the care of their NHS physicians, while benefiting from Fresenius Medical Care's dedication, proven experience and expertise in hemodialysis.
This pioneering program – part of the British Government's involvement in independent sector partnerships to deliver more choice and faster treatment to NHS patients – will see new renal dialysis units developed and existing hospital facilities extended. While new satellite centers will open the door to more patients and increase capacity, investment will also result in the installation of state-of-the art equipment and the latest technology, as well as the staff training and education.
Dr. Emanuele Gatti, Chief Executive Officer for the regions Europe, Middle East and Africa, commented: "We are very honored that Fresenius Medical Care has been chosen to work in partnership with the NHS to manage this innovative renal services program in the United Kingdom. We now have an outstanding opportunity to improve capacity for hemodialysis patients in the UK and provide easier access to services. Patients and the healthcare system at large can clearly benefit from our global expertise in renal care and our fully vertically integrated business model."
According to Kidney Research UK, a charity funding research that focuses on kidney disease, between 600 to 800 patients per one million population need renal replacement therapy in form of dialysis or transplant for survival. Kidney Research expects the number of end-stage renal disease (ESRD) patients to increase by about 5% annually.
The Company's subsidiary Fresenius Medical Care Renal Services UK is the leading provider of dialysis services to the NHS in the UK, operating 41 partnership dialysis units that provide chronic dialysis treatment for about 2,800 NHS patients.
Fresenius Medical Care is the world's largest integrated provider of products and services for individuals undergoing dialysis because of chronic kidney failure, a condition that affects more than 1,500,000 individuals worldwide. Through its network of 2,221 dialysis clinics in North America, Europe, Latin America, Asia-Pacific and Africa, Fresenius Medical Care provides dialysis treatment to 172,227 patients around the globe. Fresenius Medical Care is also the world's leading provider of dialysis products such as hemodialysis machines, dialyzers and related disposable products. Fresenius Medical Care is listed on the Frankfurt Stock Exchange (FME, FME3) and the New York Stock Exchange (FMS, FMS/P).
For more information about Fresenius Medical Care visit the Company's website at www.fmc-ag.com.
This release contains forward-looking statements that are subject to various risks and uncertainties. Actual results could differ materially from those described in these forward-looking statements due to certain factors, including uncertainties in the development of the home dialysis market, the ability to develop and commercialize technological innovations, changes in business, economic and competitive conditions, regulatory reforms, foreign exchange rate fluctuations, uncertainties in litigation or investigative proceedings, and the availability of financing. These and other risks and uncertainties are detailed in Fresenius Medical Care AG & Co. KGaA's reports filed with the U.S. Securities and Exchange Commission. Fresenius Medical Care AG & Co. KGaA does not undertake any responsibility to update the forward-looking statements in this release.
Fresenius Medical Care AG & Co. KGaA ("the Company") today announced that its wholly-owned subsidiary, FMC Finance III S.A. had extended the expiration date of its Exchange Offer to the holders of its 6-7/8% Senior Notes due 2017. The Notes will be unconditionally guaranteed by the Company, Fresenius Medical Care Holdings, Inc. and Fresenius Medical Care Deutschland GmbH. The Exchange Offer to holders of the Senior Notes will now expire at 5:00 p.m., New York City time, on February 7, 2008.
FMC Finance III S.A. is offering to exchange up to $500,000,000 aggregate principal amount of 6-7/8% Senior Notes due 2017 which have been registered under the Securities Act of 1933 ("New Notes") for a like principal amount of its outstanding 6-7/8% Senior Notes due 2017 ("Old Notes"). The Exchange Offer was originally scheduled to expire at midnight, New York time, on January 24, 2008.
At the close of business on January 24, 2008, approximately $499,885,000 out of $500,000,000 aggregate principal amount of Old Notes, including $8,785,000 aggregate principal amount tendered by guaranteed delivery procedures, have been received by U.S. Bank National Association, the Exchange Agent for the Exchange Offer.
The terms of the New Notes are identical in all material respects to the terms of the Old Notes, except for the elimination of certain transfer restrictions, registration rights and the conditional right to receive additional interest. The Exchange Offer is being made for all outstanding Old Notes and is not conditioned on any minimum principal amount of Old Notes being tendered for exchange.
Fresenius Medical Care is the world's largest integrated provider of products and services for individuals undergoing dialysis because of chronic kidney failure, a condition that affects more than1,500,000 individuals worldwide. Through its network of 2,221 dialysis clinics in North America, Europe, Latin America, Asia-Pacific and Africa, Fresenius Medical Care provides dialysis treatment to 172,227 patients around the globe. Fresenius Medical Care is also the world's leading provider of dialysis products such as hemodialysis machines, dialyzers and related disposable products. Fresenius Medical Care is listed on the Frankfurt Stock Exchange (FME, FME3) and the New York Stock Exchange (FMS, FMS/P).
For more information about Fresenius Medical Care, visit the Company's website at www.fmc-ag.com.
This release contains forward-looking statements that are subject to various risks and uncertainties. Actual results could differ materially from those described in these forward-looking statements due to certain factors, including uncertainties in the development of the home dialysis market, the ability to develop and commercialize technological innovations, changes in business, economic and competitive conditions, regulatory reforms, foreign exchange rate fluctuations, uncertainties in litigation or investigative proceedings, and the availability of financing. These and other risks and uncertainties are detailed in Fresenius Medical Care AG & Co. KGaA's reports filed with the U.S. Securities and Exchange Commission. Fresenius Medical Care AG & Co. KGaA does not undertake any responsibility to update the forward-looking statements in this release.
Fresenius Medical Care AG & Co. KGaA ("the Company") today announced that the Portuguese Ministry of Health and Anadial, the national association of privately run dialysis centers, agreed on a new reimbursement model for ambulatory care to hemodialysis patients. The new model "Comprehensive Price Payment" is an integrated and quality-driven approach that bundles a variety of dialysis related services and products. That requires the implementation and functioning of an integrated disease management model in order to achieve, simultaneously, health benefits, quality improvement and system rationalization. Including the new additional services in this reimbursement model, the Company expects the reimbursement rate to increase by about 50%.
The "Comprehensive Price Payment" model will include all necessary dialysis services, the deployment of dialysis-related products, laboratory services and other complementary medical tests and the administration of renal drugs for anemia management, bone management, blood pressure and cardiovascular control as well as vitamins.
The new reimbursement structure will provide for payment of a national reimbursement rate per week per patient. The main characteristic is that the amount of this reimbursement will directly depend on the fulfillment of certain treatment results and quality control parameters with the dialysis services provided. The therapeutic goals include, among others, the adequacy of dialysis, targets for hemoglobin levels, bone metabolism status, water quality as well as outcome measures such as mortality rate and hospitalization days. These goals mirror the good practices guidelines, both national and international, for dialysis care to patients, which will serve as support for contractual monitoring. The establishment of auditing, information, monitoring, attendance and evaluation mechanisms is a prerequisite for a participating dialysis provider.
Furthermore, the Portuguese Ministry of Health and Anadial agreed to continuously develop studies that will examine the inclusion of other modalities and treatment components such as the construction and maintenance of vascular access, hospitalization and patient transport into the comprehensive price payment.
The Company aims to fully adapt its dialysis care model to the new reimbursement structure for hemodialysis patients within its own network as of March 1, 2008.
Fresenius Medical Care Portugal is the largest private dialysis provider with annual revenues of approximately $128 million in 2007 for patient care and dialysis products. At the end of 2007, the Company provided dialysis treatment for about 4,100 patients in 33 dialysis clinics. According to internal estimates about 1,300 patients per one million population need renal replacement therapy in the form of dialysis or transplantation for survival. The number of dialysis patients is expected to increase by about 4% annually.
Ben Lipps, Chief Executive Officer of Fresenius Medical Care, commented: "Portugal is the first European country opting for a bundled reimbursement model in dialysis. The introduction of a bundled rate for the treatment of hemodialysis patients in Portugal equally benefits all those involved: the quality of life of dialysis patients can be significantly improved, health care spending for social systems and insurers remains controllable and dialysis companies gain the freedom to manage their cost structure while maximizing patient services. With our fully vertically integrated business model that includes patient care and dialysis products, Fresenius Medical Care is very well positioned to be the partner-of-choice to tackle the future challenges with growing patient numbers and – at the same time – financial burdens to social systems that derive from a disease like chronic renal failure."
Fresenius Medical Care is the world's largest integrated provider of products and services for individuals undergoing dialysis because of chronic kidney failure, a condition that affects more than1,500,000 individuals worldwide. Through its network of 2,221 dialysis clinics in North America, Europe, Latin America, Asia-Pacific and Africa, Fresenius Medical Care provides dialysis treatment to 172,227 patients around the globe. Fresenius Medical Care is also the world's leading provider of dialysis products such as hemodialysis machines, dialyzers and related disposable products. Fresenius Medical Care is listed on the Frankfurt Stock Exchange (FME, FME3) and the New York Stock Exchange (FMS, FMS/P).
For more information about Fresenius Medical Care, visit the Company's website at www.fmc-ag.com.
This release contains forward-looking statements that are subject to various risks and uncertainties. Actual results could differ materially from those described in these forward-looking statements due to certain factors, including uncertainties in the development of the home dialysis market, the ability to develop and commercialize technological innovations, changes in business, economic and competitive conditions, regulatory reforms, foreign exchange rate fluctuations, uncertainties in litigation or investigative proceedings, and the availability of financing. These and other risks and uncertainties are detailed in Fresenius Medical Care AG & Co. KGaA's reports filed with the U.S. Securities and Exchange Commission. Fresenius Medical Care AG & Co. KGaA does not undertake any responsibility to update the forward-looking statements in this release.
Summary Fourth Quarter 2007
- Net revenue : $ 2,569 million, + 9%
- Operating income (EBIT): $ 428 million, + 21%
- Net income: $ 197 million, + 30%
- Earnings per share: $ 0.67, + 29%
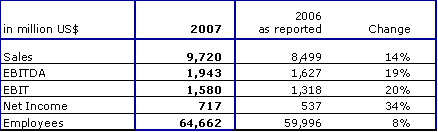
Summary Full Year 2007
- Net revenue : $ 9,720 million, + 14%
- Operating income (EBIT): $ 1,580 million, + 20%
- Net income: $ 717 million, + 34%
- Earnings per share: $ 2.43, + 33%
Dividend Proposal
- Ordinary Share: € 0.54, + 15%
- Preference Share: € 0.56, + 14%
Fresenius Medical Care AG & Co. KGaA ("the Company"), the world's largest provider of Dialysis Products and Services, today announced its results for the fourth quarter and full year of 2007.
Fourth Quarter 2007:
Revenue
Net revenue for the fourth quarter 2007 increased by 9% to $2,569 million (6% at constant currency) compared to the fourth quarter 2006. Organic revenue growth worldwide was 4%. Dialysis Services revenue grew by 6% to $1,856 million (4% at constant currency) in the fourth quarter of 2007. Dialysis Product revenue increased by 18% to $713 million (10% at constant currency) in the same period.
North America revenue increased by 3% to $1,706 million. Dialysis Services revenue grew by 1% to $1,526 million. Excluding effects of the divestiture of the perfusion business, Dialysis Service revenue increased by 3%. Average revenue per treatment for the U.S. clinics decreased by 1% to $325 in the fourth quarter 2007 compared to $328 for the same quarter in 2006. At the same time, due to extremely good cost containment, the comparable costs per treatment decreased by 2% to $266 per treatment also contributing to a further margin expansion of 70 basis points in North America. Dialysis Product revenue increased by 17% to $180 million again well above market led by strong sales of our 2008K hemodialysis machines, peritoneal dialysis products and the phosphate binding drug PhosLo.
International revenue was $863 million, an increase of 24% (12% at constant currency) compared to the fourth quarter of 2006. Organic revenue growth of the international segment was 7%. Dialysis Services revenue reached $331 million, an increase of 35% (22% at constant currency). Dialysis Product revenue rose by 18% to $533 million (7% at constant currency), led by strong peritoneal dialysis products and dialyzers.
Earnings
Operating income (EBIT) increased by 21% to $428 million compared to $354 million in the fourth quarter 2006. Operating income for the fourth quarter 2006 includes costs of $25 million related to restructuring costs and in-process R&D. Excluding these effects, operating income for the fourth quarter 2007 increased by 13%. The operating margin improved from 16.1% in the fourth quarter 2006 excluding the one-time costs to 16.6% in 2007.
| Operating income (EBIT) before one-time items Three months ended December 31 (in US-$ million) |
2007
|
2006
|
% Change
|
| Operating income (EBIT) |
428
|
354
|
+ 21%
|
| Cost of restructuring and in-process R&D |
-
|
25
|
-
|
| Operating income (EBIT) before one-time items |
428
|
379
|
+ 13%
|
In North America, the operating margin increased from 17.1% (excluding the effects of one-time items) by 70 basis points to 17.8% due to the new PhosLo business, higher product sales and decreased operating costs per treatment. In the International segment, the operating margin also increased by 70 basis points to 18.4% mainly due to higher growth in the emerging markets and increased efficiencies.
Net interest expense for the fourth quarter 2007 was $90 million compared to $96 million in the same quarter of 2006. This positive development was mainly attributable to lower average interest rates and a lower debt level.
Income tax expense was $135 million for the fourth quarter of 2007 compared to $99 million in the fourth quarter of 2006, reflecting effective tax rates of 39.8% and 38.5%, respectively.
Net income for the fourth quarter 2007 was $197 million, an increase of 30%. Net income increased by 16% when compared to the fourth quarter 2006 excluding the effects of one-time items in 2006.
| Net income before one-time items Three months ended December 31 (in US-$ million) |
2007
|
2006
|
% Change
|
| Net income |
197
|
152
|
+ 30%
|
| Cost of restructuring and in-process R&D |
-
|
18
|
|
| Net income before one-time items |
197
|
170
|
+ 16%
|
Earnings per share (EPS) for the fourth quarter of 2007 rose by 29% to $0.67 per ordinary share compared to $0.52 for the fourth quarter of 2006. The weighted average number of shares outstanding for the fourth quarter of 2007 was approximately 296.3 million shares compared to 295.0 million shares for the fourth quarter of 2006. The increase in shares outstanding resulted from stock option exercises in 2007.
Cash Flow
In the fourth quarter of 2007, the Company generated $309 million in cash from operations, representing approximately 12% of revenue, clearly ahead of our target. The extremely strong cash flow generation was primarily supported by higher earnings and a stable working capital.
A total of $184 million was spent for capital expenditures, net of disposals. Free Cash Flow before acquisitions was $125 million compared to $266 million in the fourth quarter of 2006 on a reported basis. A total of $118 million in cash was used for acquisitions.
Full Year 2007:
The operations of Renal Care Group (RCG) are included in the Company's consolidated statements of income and cash flows from April 1, 2006, therefore, the current results for the full year 2007 are not directly comparable with the results of the full year 2006.
Revenue and Earnings
Net revenue for 2007 was $9,720 million, up 14% from 2006. At constant currency, net revenue rose by 12%. Organic growth was 6% in 2007.
Operating income (EBIT) increased by 20% to $1,580 million compared to $1,318 million in 2006. Operating income for the full year 2006 includes cost of $37 million as a result of restructuring, the transformation of the Company's legal form and in-process R&D, and a gain from the clinic divestitures of $40 million.
Excluding these items, operating income for 2007 increased also by 20%. This performance resulted in an operating margin of 16.3% compared to 15.5% for the year 2006.
| Operating income (EBIT) before one-time items Twelve months ended December 31 (in US-$ million) |
2007
|
2006
|
% Change
|
| Operating income (EBIT) |
1,580
|
1,318
|
+ 20%
|
| Cost of restructuring, transformation and in-process R&D |
-
|
37
|
|
| Gain from divestiture |
-
|
(40)
|
|
| Operating income (EBIT) before one-time items |
1,580
|
1,315
|
+ 20%
|
Net interest expense for the full year 2007 was $371 million compared to $351 million in 2006. The increase was mainly the result of additional interest expense partially offset by the write-off in 2006 of deferred financing costs related to the 2003 senior credit facility of $15 million, both in conjunction with the financing of the RCG acquisition.
Income tax expense was $466 million for the full year compared to $413 million in 2006, reflecting tax rates of 38.5% and 42.8%, respectively. The tax rate for 2006 was impacted by tax payments in the U.S mainly related to the gain on the divestiture of dialysis clinics in the U.S. Excluding this impact, the effective tax rate for 2006 was at 39.8%.
For the full year 2007, net income was $717 million, up 34% from 2006. Net income for 2007 increased by 25% compared to 2006 excluding the effects of one-time items in 2006.
| Net income before one-time items Twelve months ended December 31 (in US-$ million) |
2007
|
2006
|
% Change
|
| Net income |
717
|
537
|
+ 34%
|
| Cost of restructuring, transformation and in-process R&D |
-
|
24
|
|
| Write-off FME prepaid financing fees |
-
|
9
|
|
| Loss from divestiture |
-
|
4
|
|
| Net income before one-time items |
717
|
574
|
+ 25%
|
For the full year 2007, earnings per ordinary share rose by 33% to $2.43. The weighted average number of shares outstanding during 2007 was approximately 295.7 million.
Cash Flow
Cash from operations during the full year 2007 was $1,200 million compared to $908 million for 2006 on a reported basis. Excluding the effects of one-time items, cash from operations was $1,106 million for 2006. The increase compared to prior year was mainly due to increased earnings.
A total of $549 million was used for capital expenditures, net of disposals. Free Cash Flow before acquisitions for 2007 was $651 million compared to $458 million in 2006. The underlying Free Cash Flow before acquisitions and the effects of one-time items for 2006 was $656 million. A total of $228 million in cash was used for acquisitions, net of divestitures. Free Cash Flow after acquisitions for the full year 2007 was $423 million.
Please refer to the PDF-file for a complete overview on the fourth quarter and the full year of 2007.
Patients – Clinics – Treatments
As of December 31, 2007, Fresenius Medical Care treated 173,863 patients worldwide, which represents a 6% increase in patients compared to last year. North America provided dialysis treatments for 121,431 patients, an increase of 3%. Including 33 clinics managed by Fresenius Medical Care North America, the number of patients in North America was 123,273. The International segment served 52,432 patients, an increase of 15% over last year.
As of December 31, 2007, the Company operated a total of 2,238 clinics worldwide. This is comprised of 1,602 clinics in North America, an increase of 3%, and 636 clinics in the International segment, an increase of 16%.
Fresenius Medical Care delivered approximately 26.44 million dialysis treatments worldwide during 2007. This represents an increase of 11% year over year. North America accounted for 18.45 million treatments, an increase of 9%, and the International segment delivered 7.99 million treatments, an increase of 16% over last year.
Employees
As of December 31, 2007, Fresenius Medical Care had 61,406 employees (full-time equivalents) worldwide compared to 56,803 employees at the end of 2006. The increase of 4,603 employees is primarily due to continued organic growth in the U.S. and acquisitions in Asia.
Dividends
The Company will continue to follow an earnings-driven dividend policy. For the eleventh consecutive year, shareholders can expect to receive an increased annual dividend for the fiscal year 2007. At the Annual General Meeting to be held on May 20, 2008, shareholders will be asked to approve a dividend of €0.54 per ordinary share, an increase of 15% from 2006 (€0.47) and €0.56 per preference share, an increase of 14% from 2006 (€0.49).
Debt/EBITDA Ratio
The ratio of debt to Earnings before Interest, Taxes and Amortization (EBITDA) decreased from 3.23 at the end of 2006 to 2.84 at the end of 2007.
Rating
There have been no rating changes in the fourth quarter 2007, Standard & Poor's Ratings Services rates the Company's corporate credit rating as 'BB' with a ‘stable' outlook.
Moody's rates the Company's corporate credit rating as ‘Ba2' with a ‘positive' outlook.
Refinancing of Trust Preferred Securities
At the beginning of February, we refinanced our Capital Trust II and III Trust Preferred Securities. They were mandatorily redeemable after a period of ten years, expiring on February 1, 2008. The stated amount of Capital Trust II was $450 million, with a fixed interest rate of 7 7/8%; the stated amount of Capital Trust III totaled DM300 million, with an interest rate of 7 3/8%. Fresenius Medical Care's existing financing facilities were utilized to affect the refinancing.
Outlook for 2008
For the full year 2008, the Company expects to achieve revenue of more than $10.4 billion, an increase of more than 7%.
Net income is expected to be between $805 million and $825 million in 2008, an increase of 12% to 15%.
The Company expects to spend $650 to $750 million on capital expenditures and $150 to $250 million on acquisitions. The debt/EBITDA ratio is expected to decrease below 2.8 by the end of 2008.
For 2010, Fresenius Medical Care continues to expect revenue of more than $11.5 billion. Earnings after tax are projected to grow in the low- to mid-teens per year.
Ben Lipps, Chief Executive Officer of Fresenius Medical Care, commented: "Our excellent performance in 2007 achieving record revenue and earnings for the year reflects the strong demand for our products and services worldwide. Both, the North American segment and the International segment contributed to this outstanding performance. Overall, we continued to grow above market in our global product business and are the leading provider of dialysis services in all four major regions in the world. We are pleased to have again exceeded our guidance in 2007 and are proposing to deliver our eleventh consecutive dividend increase to our shareholders. We have made good progress on our growth initiatives and with our global presence, we are confident to continue our strong performance in 2008."
Video Webcast
Fresenius Medical Care will hold an analyst meeting at its headquarters in Bad Homburg, Germany, to discuss the results of the fourth quarter and the full year of 2007 on Wednesday, February 20, 2008, at 3.15pm CET / 9.15am EST. The Company invites investors to view the live webcast of the meeting at the Company's website www.fmc-ag.com in the "Investor Relations" section. A replay will be available shortly after the meeting.
Fresenius Medical Care is the world's largest integrated provider of products and services for individuals undergoing dialysis because of chronic kidney failure, a condition that affects more than 1,600,000 individuals worldwide. Through its network of 2,238 dialysis clinics in North America, Europe, Latin America, Asia-Pacific and Africa, Fresenius Medical Care provides dialysis treatment to 173,863 patients around the globe. Fresenius Medical Care is also the world's leading provider of dialysis products such as hemodialysis machines, dialyzers and related disposable products. Fresenius Medical Care is listed on the Frankfurt Stock Exchange (FME, FME3) and the New York Stock Exchange (FMS, FMS/P).
For more information about Fresenius Medical Care visit the Company's website at www.fmc-ag.com.
This release contains forward-looking statements that are subject to various risks and uncertainties. Actual results could differ materially from those described in these forward-looking statements due to certain factors, including changes in business, economic and competitive conditions, regulatory reforms, foreign exchange rate fluctuations, uncertainties in litigation or investigative proceedings, and the availability of financing. These and other risks and uncertainties are detailed in Fresenius Medical Care AG & Co. KGaA's reports filed with the U.S. Securities and Exchange Commission. Fresenius Medical Care AG & Co. KGaA does not undertake any responsibility to update the forward-looking statements in this release.
Fresenius Medical Care
Statement of Earnings
see PDF-file
- Sales: € 11.4 billion, + 5 % at actual rates, + 10 % in constant currency
- EBIT: € 1.6 billion, + 11 % at actual rates, + 17 % in constant currency
- Net income: € 410 million, + 24 % at actual rates, + 28 % in constant currency
- All financial targets met or exceeded
- Double-digit EBIT growth in all business segments
- Strengthened market position through targeted acquisitions
- 15th consecutive dividend increase
Dividend increase of ~15 % per share proposed
Based on the excellent financial results the Management Board will propose to the Supervisory Board a dividend increase of ~15 % to € 0.66 per ordinary share (2006: € 0.57) and € 0.67 per preference share (2006: € 0.58). The corresponding total dividend distribution amounts to € 103.2 million (2006: € 88.8 million).
Positive outlook for 2008: Substantial sales and earnings growth expected
For 2008, Fresenius Group projects further improvements in its financial results: Group sales are expected to grow by 8 to 10 % in constant currency. Net income is expected to increase by 10 to 15 % in constant currency. All business segments are expected to contribute to this growth.
Investments in property, plant and equipment and in intangible assets are planned to increase from € 705 million in 2007 to ~€ 750 million.
Strong sales growth across all business segments and regions
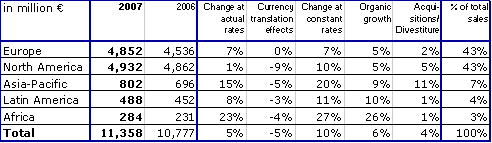
Group sales increased by 10 % in constant currency and by 5 % at actual rates to € 11,358 million (2006: € 10,777 million). Organic sales growth was 6 %. Acquisitions contributed a further 6 %. Divestitures reduced sales growth by 2 %. Currency translation had a negative impact of 5 %. This is mainly attributable to the average US dollar rate depreciating 9 % against the Euro.
Sales growth in the business segments was affected as follows:
In Europe sales grew by 7 % in constant currency with organic sales growth contributing 5 %. In North America sales grew by 10 % in constant currency due to the full-year Renal Care Group consolidation and an organic growth rate of 5 %. Strong growth rates were achieved in the emerging markets with organic growth of 9 % in Asia-Pacific, 10 % in Latin America and 26 % in Africa.
Excellent earnings growth and strong margin improvement
Group EBITDA increased by 15 % in constant currency and by 10 % at actual rates to € 2,030 million (2006: € 1,843 million). Group operating income (EBIT) grew by 17 % in constant currency and by 11 % at actual rates to € 1,609 million (2006: € 1,444 million). The Group's EBIT margin improved by 80 basis points to 14.2 % (2006: 13.4 %).
Group net interest was € -368 million (2006: € -395 million, including one-time expenses of € 30 million for the early refinancing of Group debt).
The tax rate was 36.1 % (2006: 39.5 %; adjusted for the tax expense related to the divestiture of US dialysis clinics: 37.2 %).
Minority interest increased to € 383 million (2006: € 305 million), of which 92 % was attributable to the minority interest in Fresenius Medical Care.
Group net income grew strongly by 28 % in constant currency and by 24 % at actual rates to € 410 million (2006: € 330 million, including one-time expenses of € 22 million). Earnings per ordinary share were € 2.64 and earnings per preference share were € 2.65 (2006 adjusted for the February 2007 share split: ordinary share € 2.15, preference share € 2.16). This represents an increase of 23 % for both share classes.
Investments in property, plant and equipment at high level
Fresenius Group spent € 705 million for property, plant and equipment and intangible assets (2006: € 600 million). The increase is mainly attributable to the business segments Fresenius Medical Care and Fresenius Helios. Acquisition spending was € 613 million (2006: € 3,714 million). All business segments strengthened their market position through targeted acquisitions.
Strong cash flow
Operating cash flow increased by 23 % to € 1,296 million (2006: € 1,052 million). Key driver was the strong increase in earnings. Cash flow margin was 11.4 % (2006: 9.8 %). Cash flow before acquisitions and dividends increased by 31 % to € 630 million (2006: € 481 million). Free cash flow after acquisitions (€ 392 million) and dividends (€ 205 million) was € 33 million (2006: € -2,909 million).
Solid balance sheet structure: Leverage ratio improved
Fresenius Group's total assets increased by 8 % in constant currency and by 2 % at actual rates to € 15,324 million (December 31, 2006: € 15,024 million). Current assets increased by 5 % to € 4,291 million (December 31, 2006: € 4,106 million). Non-current assets were € 11,033 million (December 31, 2006: € 10,918 million).
Shareholders' equity including minority interest grew by 6 % to € 6,059 million (December 31, 2006: € 5,728 million). The equity ratio (including minority interest) was 39.5 % (December 31, 2006: 38.1 %).
Group debt decreased by 3 % at actual rates to € 5,699 million (December 31, 2006: € 5,872 million). In constant currency, Group debt increased by 3 %. The net debt/EBITDA ratio improved to 2.6, well below the level of 3.0 as of December 31, 2006.
Number of employees increased
As of December 31, 2007, Fresenius increased the number of its employees by 9 % to 114,181 (December 31, 2006: 104,872). The increase is mainly attributable to the business segments Fresenius Medical Care and Fresenius Helios.
Fresenius Biotech
Fresenius Biotech develops innovative therapies with trifunctional antibodies for the treatment of cancer. In the field of polyclonal antibodies, Fresenius Biotech has successfully marketed ATG-Fresenius S for many years. ATG-Fresenius S is an immunosuppressive agent used to prevent and treat graft rejection following organ transplantation.
Following the successful completion of the phase II/III study with Removab® in the indication malignant ascites, Fresenius Biotech dispatched the marketing authorization application to the European Medicines Agency (EMEA) in December 2007. The company applies for the EU authorization of Removab® for the intraperitoneal treatment of malignant ascites in patients with epithelial cancers where no standard therapy is available or no longer feasible. EMEA started the scientific evaluation of the dossier at the end of January 2008. In additional phase II studies Fresenius Biotech is focusing on the use of Removab® for solid tumors in the indications ovarian cancer and gastric cancer.
For the future marketing of Removab® in the USA and Japan, Fresenius Biotech is in discussions with potential partners.
Phase II studies with the antibody Rexomun® (ertumaxumab) in the indication breast cancer are ongoing.
In 2007, Fresenius Biotech's operating income (EBIT) was € -50 million (2006: € -45 million). For 2008, Fresenius Biotech expects an EBIT of approximately € -50 million.
The Business Segments
Fresenius Medical Care
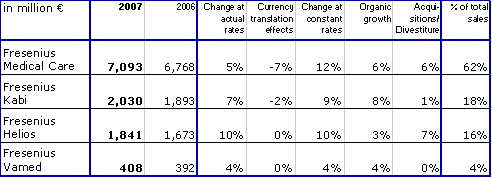
Fresenius Medical Care is the world's leading provider of services and products for patients with chronic kidney failure. As of December 31, 2007, Fresenius Medical Care was serving 173,863 patients in 2,238 dialysis clinics.
- Sales increased by 14 % to US$ 9.7 billion
- Net income well above guidance
- Outlook 2008: Sales growth of more than 7 % and net income growth
- of 12 to 15 % expected
In 2007, Fresenius Medical Care achieved excellent sales growth of 14 % to US$ 9,720 million (2006: US$ 8,499 million). This was mainly driven by organic growth of 6 % and by the full-year consolidation of Renal Care Group. Sales in dialysis care increased by 13 % to US$ 7,213 million (2006: US$ 6,377 million). In dialysis products, sales grew by 18 % to US$ 2,507 million (2006: US$ 2,122 million).
In North America sales increased by 11 % to US$ 6,663 million (2006: US$ 6,025 million). Sales outside North America ("International" segment) grew by 24 % (in constant currency: 15 %) to US$ 3,057 million (2006: US$ 2,474 million). Strong sales growth in constant currency was achieved in Europe (+9 %), Latin America (+14 %), and the Asia-Pacific region (+40 %).
EBIT rose by 20 % to US$ 1,580 million (2006: US$ 1,318 million). The EBIT margin was 16.3 % (2006: 15.5 %). Net income increased by 34 % to US$ 717 million (2006: US$ 537 million, including one-time expenses of US$ 37 million).
In November 2007, Fresenius Medical Care announced the acquisition of Renal Solutions, Inc. (RSI). With the RSI transaction, Fresenius Medical Care is acquiring a key technology for the expansion of home hemodialysis.
For 2008, Fresenius Medical Care expects to achieve revenue of more than US$ 10.4 billion, an increase of more than 7 %. Net income is expected to be between US$ 805 million and US$ 825 million, an increase of 12 % to 15 %.
For further information, please see Fresenius Medical Care's press release at www.fmc-ag.com.
Fresenius Kabi
Fresenius Kabi offers infusion therapies and clinical nutrition for seriously and chronically ill patients in the hospital and out-patient environments. The company is also a leading provider of transfusion technology products.
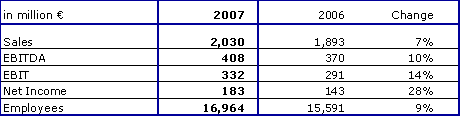
- Sales exceed 2 billion euros for the first time
- Targeted acquisitions strengthen market position
- Outlook 2008: Strong sales growth and EBIT margin of around 16.5 %
Fresenius Kabi increased sales by 7 % to € 2,030 million (2006: € 1,893 million). The company achieved excellent organic growth of 8 %, at the upper end of the guidance of 6 to 8 %. Acquisitions contributed 1 % to sales. Currency translation effects had a negative impact of 2 %. This was mainly due to the depreciation of currencies in South Africa, China, Mexico and Canada.
Organic sales growth in Europe (excluding Germany) was 5 %. In Germany organic sales growth was 2 %. In the Asia-Pacific region Fresenius Kabi achieved significant organic sales growth of 22 %. Organic sales growth in Latin America was 9 % and in other regions 10 %.
Fresenius Kabi continued its excellent earnings growth in 2007. EBIT grew by 14 % to € 332 million (2006: € 291 million). The EBIT margin improved by 100 basis points to 16.4 % (2006: 15.4 %). Fresenius Kabi reported strong growth in net income of 28 % to € 183 million (2006: € 143 million, including one-time expenses for early debt refinancing of € 11 million).
In the fourth quarter of 2007, Fresenius Kabi announced acquisitions to strengthen its business activities especially in the fields of clinical nutrition and intravenously administered generic drugs (I.V. drugs). Fresenius Kabi acquired from Nestlé S.A. the enteral nutrition businesses in France (Novartis Nutrition) and in Spain (Nestlé España). In addition, Fresenius Kabi acquired the Chilean company Laboratorio Sanderson S.A. and the Italian company Ribbon S.r.L. Aggregate sales of the three acquired businesses was about € 128 million in 2007.
Fresenius Kabi expects to continue its positive financial performance in 2008. The company targets sales growth in constant currency of 12 to 15 %. Organic growth is expected to contribute 7 % to this target. Strong growth is anticipated in particular from the Asia-Pacific and Latin America regions. Further, Fresenius Kabi forecasts an EBIT margin of around 16.5 %. It is anticipated that the recent acquisitions will initially contribute to Fresenius Kabi's EBIT at a margin below par, also due to amortization of intangible assets. Adjusted for the recent acquisitions, Fresenius Kabi's EBIT margin is expected to progress into the range of 16.5 to 17 %.
Fresenius ProServe
As from January 1, 2008, the former business segment Fresenius ProServe has been replaced by the two business segments Fresenius Helios and Fresenius Vamed. These two businesses had previously made up the business segment Fresenius ProServe. The financial results of Fresenius Helios and Fresenius Vamed are already presented separately on the following pages for the full-year 2007.
The financial performance at Fresenius ProServe was as follows:
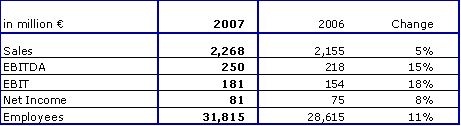
Sales grew by 5 % to € 2,268 million (2006: € 2,155 million). Organic growth was 3 %. EBIT increased by 18 % to € 181 million (2006: € 154 million). The EBIT margin improved to 8.0 % (2006: 7.1 %).
Organic sales growth guidance of 2 - 3 % and EBIT projection of > € 170 million was fully achieved.
The subsidiaries Pharmaplan and Pharmatec were divested and deconsolidated as from January 1, 2007, and June 30, 2007, respectively.
Fresenius Helios
Fresenius Helios is one of the largest private hospital operators in Germany. The HELIOS Kliniken Group owns 60 hospitals, including five maximum care hospitals in Erfurt, Berlin-Buch, Wuppertal, Schwerin and Krefeld. HELIOS treats about 500,000 inpatients per year at its clinics and has a total of approximately 17,200 beds.
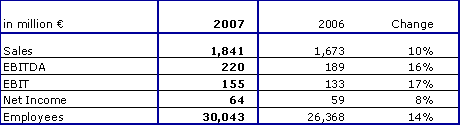
- Sales and earnings substantially increased
- Expansion in the German hospital market continued
- Outlook 2008: Sales of more than 2 billion euros expected
Fresenius Helios increased sales by 10 % to € 1,841 million (2006: € 1,673 million), and achieved very good organic growth of 3 %. Acquisitions contributed 9 %, divestitures reduced sales growth by 2 %.
EBIT increased by 17 % to € 155 million (2006: € 133 million). The EBIT margin improved by 50 basis points to 8.4 %. Fresenius Helios achieved this very good result despite a number of external negative factors: the increase in value-added tax, wage tariff increases, and the 0.5 % budget cut for the stabilization of public health costs all affected earnings. Net income improved by 8 % to € 64 million (2006: € 59 million).
In the fourth quarter of 2007, Fresenius Helios acquired 74.9 % of Krefeld Municipal Hospitals (Krefeld and Hüls). Both hospitals together have approximately 3,300 employees and achieved sales of about € 175 million in 2006. The hospitals are consolidated in the Group's balance sheet as of December 31, 2007.
The outlook for the full year 2008 remains very positive. Fresenius Helios expects to achieve sales of more than € 2,050 million. EBIT is projected to increase to € 160 to 170 million, despite the initially negative contribution of the Krefeld Municipal Hospitals.
Fresenius Vamed
Fresenius VAMED offers engineering and services for hospitals and other health care facilities.

- Order intake and order backlog at all-time high
- Acquisition in the service business for hospitals
- Outlook 2008: Sales and EBIT growth of 5 – 10 % expected
Fresenius Vamed achieved sales growth of 4 % to € 408 million (2006: € 392 million). The project business generated sales of € 259 million (2006: € 249 million), sales in the service business was € 149 million (2006: € 143 million), an increase of 4 % in each segment.
EBIT was € 26 million (2006: € 23 million). The EBIT margin improved to 6.4 % (2006: 5.9 %). Net income increased by 15 % to € 23 million (2006: € 20 million).
Order intake in the project business grew by 17 % to € 395 million (2006: € 337 million). In the fourth quarter of 2007, order intake rose by 70 % compared to the same quarter of the previous year and reached € 173 million. Order backlog as of December 31, 2007, was € 510 million (December 31, 2006: € 387 million).
In February 2008, Fresenius Vamed announced that it had signed an agreement to acquire the hospital planning, consulting and service company HERMED in Germany. Both regionally and strategically, HERMED fits perfectly to the business of VAMED. VAMED concentrates on larger hospitals whereas HERMED focuses on smaller and medium-sized health care facilities. HERMED achieved sales of around € 12 million in 2007.
In 2008, Fresenius Vamed expects to achieve sales growth and an increase in EBIT of 5 to 10 %.
Press Conference and Video Webcast
As part of the publication of the results for fiscal year 2007, a press conference will be held at the Fresenius headquarters in Bad Homburg on February 20, 2008 at 10 a.m. CET. All journalists are cordially invited to follow the conference in a live broadcast over our Website. Following the meeting, a recording of the conference will be available as video-on-demand.
Annual report
The annual report 2007 will be available from March 11, 2008 at www.fresenius.com / Investor Relations / Financial reports.
Fresenius is a health care group with international operations, providing products and services for dialysis, hospital and outpatient medical care. In 2007, group sales were approx. € 11.4 billion. On December 31, 2007 the Fresenius Group had 114,181 employees worldwide.
This release contains forward-looking statements that are subject to various risks and uncertainties. Future results could differ materially from those described in these forward-looking statements due to certain factors, e.g. changes in business, economic and competitive conditions, regulatory reforms, results of clinical trials, foreign exchange rate fluctuations, uncertainties in litigation or investigative proceedings, and the availability of financing. Fresenius does not undertake any responsibility to update the forward-looking statements in this release.
Board of Management: Dr. Ulf M. Schneider (President and CEO), Rainer Baule, Dr. Francesco De Meo, Dr. Jürgen Götz, Dr. Ben Lipps, Stephan Sturm, Dr. Ernst Wastler
Supervisory Board: Dr. Gerd Krick (Chairman)
Registered Office: Bad Homburg, Germany
Commercial Register No. HRB 10660
PDF-file includes:
Fresenius Group in Figures
- Consolidated statement of income (US GAAP)
- Key figures of the balance sheet (US GAAP)
- Cash flow statement (US GAAP)
- Segment reporting by business segment Q1-4 (US GAAP)
- Segment reporting by business segment Q4 (US GAAP)
Due to strong global demand, Fresenius Medical Care is expanding the capacity for producing dialysis products at its plant in St. Wendel, Germany. The Company will invest a total of approximately €39 million over the next 12 months.
About €23 million will be invested in an additional building and two new spinning lines for hollow fibers, the key component of dialyzers (artificial kidneys). Production capacity for these fibers, which are also used to manufacture dialyzers at other locations, will increase by about 30 % at St. Wendel.
Fresenius Medical Care will invest about €16 million to increase the production capacity for bags used in peritoneal dialysis. This should increase capacity by some 25%.
At the beginning of 2007, the Company had already announced an increase to the dialyzer production capacity at the St. Wendel plant. Some €36 million were committed to the project, which will boost annual capacity for single-use dialyzers to 35 million. This equipment will be brought on line in the coming months and the two latest expansion measures announced today should be completed in spring of 2009. The committed total amount for all ongoing projects at St. Wendel is about €100 million (US$150 million).
Fresenius Medical Care currently employs about 1,700 people at the St. Wendel development and production site. A further increase in the number of employees is expected for 2008.
Dr. Ben Lipps, Chief Executive Officer of Fresenius Medical Care commented: "Demand for our innovative dialyzers and other high-quality dialysis products continues to climb. The capacity expansion at the St. Wendel manufacturing site meets this steady increase and ensures that an even larger number of patients worldwide can receive our life-saving products and therapies. The investments at St. Wendel are a commitment to Germany as an important manufacturing base for us and proof of the dedication of our employees. Their efforts over the past years have made our successful growth possible."
Fresenius Medical Care is the world's largest integrated provider of products and services for individuals undergoing dialysis becaus
e of chronic kidney failure, a condition that affects more than 1,600,000 individuals worldwide. Through its network of 2,238 dialysis clinics in North America, Europe, Latin America, Asia-Pacific and Africa, Fresenius Medical Care provides dialysis treatment to 173,863 patients around the globe. Fresenius Medical Care is also the world's leading provider of dialysis products such as hemodialysis machines, dialyzers and related disposable products. Fresenius Medical Care is listed on the Frankfurt Stock Exchange (FME, FME3) and the New York Stock Exchange (FMS, FMS/P).
For more information about Fresenius Medical Care visit the Company's website at www.fmc-ag.com.
This release contains forward-looking statements that are subject to various risks and uncertainties. Actual results could differ materially from those described in these forward-looking statements due to certain factors, including changes in business, economic and competitive conditions, regulatory reforms, foreign exchange rate fluctuations, uncertainties in litigation or investigative proceedings, and the availability of financing. These and other risks and uncertainties are detailed in Fresenius Medical Care AG & Co. KGaA's reports filed with the U.S. Securities and Exchange Commission. Fresenius Medical Care AG & Co. KGaA does not undertake any responsibility to update the forward-looking statements in this release.
Fresenius Medical Care AG & Co. KGaA ("the Company"), the world's largest provider of Dialysis Products and Services, today reports that Standard & Poor's has assigned upgraded debt and recovery ratings to the Company's unsecured debt issues. Based on a recovery analysis the rating of Trust Preferred Securities IV ($225 million) and Trust Preferred Securities V (€300 million) improved to BB from single B+. Additionally the rating of the $500 million Senior Notes due 2017 improved to BB+ from BB-. The corporate credit rating remains unchanged at BB, the outlook is stable.
Lawrence A. Rosen, Chief Financial Officer, commented: "The Company welcomes the improved ratings which reflect our strong global position in growing, non-cyclical markets of the dialysis industry. The dialysis industry is characterized by stable cash flows; most of the Company's customers have a high credit rating. Furthermore, we have a proven ability to reduce debt significantly over time. Our financial position is strong and we are confident to meet our financial targets."
| Fresenius Medical Care AG & Co. KGaA | ||||
| Facility |
Year |
Amount |
New |
Old |
| Corporate Credit Rating |
|
|
BB |
BB |
| Credit Agreement Term Loan A |
2006 |
$ 1,850 |
BBB- |
BBB- |
| Credit Agreement Term Loan B |
2006 |
$ 1,750 |
BBB- |
BBB- |
| Secured Revolving Credit Facility |
2006 |
$ 1,000 |
BBB- |
BBB- |
| Senior Notes 2007-2017 |
2007 |
$ 500 |
BB |
B+ |
| Trust Preferred Securities IV (subordinated notes) |
2001 |
$ 225 |
BB |
B+ |
| Trust Preferred Securities V (subordinated notes) |
2001 |
300 |
BB |
B+ |
Fresenius Medical Care is the world's largest integrated provider of products and services for individuals undergoing dialysis becaus
e of chronic kidney failure, a condition that affects more than 1,600,000 individuals worldwide. Through its network of 2,238 dialysis clinics in North America, Europe, Latin America, Asia-Pacific and Africa, Fresenius Medical Care provides dialysis treatment to 173,863 patients around the globe. Fresenius Medical Care is also the world's leading provider of dialysis products such as hemodialysis machines, dialyzers and related disposable products. Fresenius Medical Care is listed on the Frankfurt Stock Exchange (FME, FME3) and the New York Stock Exchange (FMS, FMS/P).
For more information about Fresenius Medical Care visit the Company's website at www.fmc-ag.com.
This release contains forward-looking statements that are subject to various risks and uncertainties. Actual results could differ materially from those described in these forward-looking statements due to certain factors, including changes in business, economic and competitive conditions, regulatory reforms, foreign exchange rate fluctuations, uncertainties in litigation or investigative proceedings, and the availability of financing. These and other risks and uncertainties are detailed in Fresenius Medical Care AG & Co. KGaA's reports filed with the U.S. Securities and Exchange Commission. Fresenius Medical Care AG & Co. KGaA does not undertake any responsibility to update the forward-looking statements in this release.
Fresenius Medical Care AG & Co. KGaA ("The Company") today announced that the United States District Court for the Northern District of California issued orders in the long standing patent case initiated by the Company to establish the invalidity of certain patents claims alleged to apply to the Company's 2008K hemodialysis machines in United States. These orders reflect another step in this lengthy legal process. The Company continues to believe with good reasons that these patents claims are invalid as determined by the U.S. Patent Office in recent reexaminations and will therefore appeal the court decision with confidence. In addition, the Company has prudently developed a back up design while this legal process continues and expects no material impact on its product business with hemodialysis machines.
The United States District Court for the Northern District of California has entered orders denying Baxter's motion for a new trial, establishing a royalty for continuing sales and deferring entry of an injunction until January 2009.
Fresenius Medical Care Holdings ("FMCH") intends to appeal to the United States Court of Appeals for the Federal Circuit for reinstatement of the original jury verdict (July 2006) of invalidity on all patent claims remaining in this case relating to touch screen interfaces for hemodialysis machines.
In June 2006, Fresenius Medical Care Holdings prevailed in a jury trial relating to patent claims regarding touch screen interfaces for hemodialysis machines when the jury found all of the patent claims asserted against FMCH to be invalid.
The trial court overturned that jury's verdict and ordered a new trial to determine damages. In October 2007 a second jury, in the trial to determine damages, rejected Baxter's demand that FMCH pay more than $149 million and instead awarded damages of $14.3 million on FMCH's sales of more than $2 billion of hemodialysis machines and disposables through October 2007.
On April 4, 2008, the trial court denied Baxter's motion to overturn the second jury's $14.3 million damage award and order a second new trial. Nevertheless, the Court entered orders establishing royalties of 10% on all sales of 2008K hemodialysis machines and 7% on sales of related disposables beginning November 7, 2007, and deferred enjoining sales of 2008K machines until after January 1, 2009.
Since the initiation of this litigation, the United States Patent and Trademark Office ("USPTO"), in re-examination proceedings, has rejected all the asserted claims of the patents as invalid and obvious. The USPTO's rejections of the two patents central to this dispute have been made final.
With several previous decisions clearly in favor of the Company, FMCH will appeal to the Court of Appeals for the Federal Circuit to have the original June 2006 jury verdict of invalidity reinstated and will seek a stay of the injunction pending resolution of the appeal.
Dr. Ben J. Lipps, Chairman of the Management Board of FMCAG KGaA commented, "We are convinced that the original verdict of invalidity was amply supported by the evidence. We note that the Unites States Patent and Trademark Office similarly found the claims to be invalid when presented with a substantial record of prior art. We are confident that based on that record the Court of Appeals will confirm the original jury verdict of invalidity. In the meantime, we have developed alternate user interface designs and with the respite given, we do not expect any material impact for our North American business. We will continue to provide to patients and providers the machines and supplies that meet our exacting standards for quality and reliability."
Fresenius Medical Care is the world's largest integrated provider of products and services for individuals undergoing dialysis becaus
e of chronic kidney failure, a condition that affects more than 1,600,000 individuals worldwide. Through its network of 2,238 dialysis clinics in North America, Europe, Latin America, Asia-Pacific and Africa, Fresenius Medical Care provides dialysis treatment to 173,863 patients around the globe. Fresenius Medical Care is also the world's leading provider of dialysis products such as hemodialysis machines, dialyzers and related disposable products. Fresenius Medical Care is listed on the Frankfurt Stock Exchange (FME, FME3) and the New York Stock Exchange (FMS, FMS/P).
For more information about Fresenius Medical Care visit the Company's website at www.fmc-ag.com.
This release contains forward-looking statements that are subject to various risks and uncertainties. Actual results could differ materially from those described in these forward-looking statements due to certain factors, including changes in business, economic and competitive conditions, regulatory reforms, foreign exchange rate fluctuations, uncertainties in litigation or investigative proceedings, and the availability of financing. These and other risks and uncertainties are detailed in Fresenius Medical Care AG & Co. KGaA's reports filed with the U.S. Securities and Exchange Commission. Fresenius Medical Care AG & Co. KGaA does not undertake any responsibility to update the forward-looking statements in this release.



