Conference Call FY/2024
Conference Call FY/2024 Presentation
The information and documents contained on the following pages of this website are for information purposes only. These materials do neither constitute an offer nor an invitation to subscribe to or to purchase securities, nor any investment advice or service, and are not meant to serve as a basis for any kind of obligation, contractual or otherwise. The securities described on the following pages have not been, and will not be, registered under the U.S. Securities Act of 1933, as amended (the "Securities Act"), and may not be offered or sold in the United States of America (the "United States") absent registration or an exemption from, or in a transaction not subject to, the registration requirements of the Securities Act. There will be no public offering of such securities in the United States or anywhere else and, if offered, any such securities will be offered and sold only (i) outside of the United States in "offshore transactions" in accordance with Regulation S of the Securities Act and (ii) in the United States to "qualified institutional buyers" (as defined in Rule 144A under the Securities Act) in transactions exempt from the registration requirements of the Securities Act.
THE FOLLOWING INFORMATION AND DOCUMENTS ARE NOT DIRECTED AT AND ARE NOT INTENDED FOR USE BY (I) PERSONS WHO ARE RESIDENTS OF OR LOCATED IN THE UNITED STATES, CANADA, AUSTRALIA, JAPAN OR SOUTH AFRICA, OR (II) PERSONS IN ANY OTHER JURISDICTION WHERE THE COMMUNICATION OR RECEIPT OF SUCH INFORMATION IS RESTRICTED IN SUCH A WAY THAT PROVIDES THAT SUCH PERSONS SHALL NOT RECEIVE IT. SUCH PERSONS, OR PERSONS ACTING FOR THE BENEFIT OF ANY SUCH PERSONS, ARE NOT PERMITTED TO VISIT THE FOLLOWING PAGES OF THE WEBSITE.
To visit the following parts of this website you must confirm that
(i) you are not a resident of or located in the United States, Canada, Australia, Japan or South Africa,
(ii) you are not a person to whom the communication of the information contained on the website is restricted,
(iii) you will not distribute any of the information and documents contained thereon to any such person, and
(iv) you are not acting for the benefit of any such person.
By clicking on the "Accept" button below, you will be deemed to have made this confirmation.
THIS ANNOUNCEMENT, INCLUDING THE INFORMATION INCLUDED HEREIN, IS RESTRICTED AND IS NOT FOR PUBLICATION, DISTRIBUTION OR RELEASE IN OR INTO THE UNITED STATES OF AMERICA, CANADA, AUSTRALIA, JAPAN, SOUTH AFRICA OR ANY OTHER JURISDICTION IN WHICH OFFERS OR SALES OF THE SECURITIES WOULD BE PROHIBITED BY APPLICABLE LAW.
THIS ANNOUNCEMENT IS FOR INFORMATION PURPOSES ONLY AND IS NOT AN OFFER OF SECURITIES IN ANY JURISDICTION.
Fresenius SE & Co. KGaA (Frankfurt/Xetra: FRE) today announced its intention to reduce its stake in Fresenius Medical Care AG ("FME").
Fresenius intends to sell approximately 10.5 million shares of FME (the "Shares"), equivalent to approximately 3.6% of FME's issued share capital, by way of an accelerated bookbuilding procedure (the "Equity Offering"). In addition, Fresenius intends to issue bonds exchangeable into ordinary Shares with approximately 10.5 million Shares underlying, equivalent to approximately 3.6% of FME's issued share capital (the "Exchangeable Bonds" and together with the Equity Offering, the "Combined Offering"). The final size of the respective instruments is to be determined following the completion of the bookbuilding process. Fresenius will retain no less than 25 per cent plus one share of FME.
Fresenius will use the proceeds in line with the #FutureFresenius strategy and the Fresenius' stated capital allocation priorities, including further strengthening the balance sheet, reducing leverage, and delivering long-term growth and shareholder value.
Following the completion of this transaction, Fresenius remains by far the largest shareholder of FME and will continue to actively support the management board through the two Fresenius representatives on the supervisory board of FME.
The placements will start immediately following this announcement and will be addressed to institutional investors only. BofA Securities Europe SA and Goldman Sachs Bank Europe SE are acting as Joint Global Coordinators and alongside BNP Paribas and Deutsche Bank Aktiengesellschaft as Joint Bookrunners on the Combined Offering, with Banco Santander, S.A. acting as Co-Lead Manager. In the context of the placements, Fresenius has agreed to a lock-up undertaking of 180 days, subject to customary exceptions.
The Exchangeable Bonds will have a maturity of 3 years, will be issued with a denomination of EUR 100,000 each at a price between 100.75% and 102.25% of their principal amount and are expected to pay no periodic interest, resulting in a yield-to-maturity of between (0.75)% and (0.25)% per annum. The exchange premium will be set at pricing and is expected to be between 25% and 30% above the placement price per Share in the Equity Offering and the Delta Placement (as defined below).
The Company has been informed by the Joint Bookrunners that the Joint Bookrunners will organize a simultaneous placement of Shares on behalf of certain subscribers of the Exchangeable Bonds who wish to sell these Shares in short sales to purchasers procured by the Joint Bookrunners in order to hedge the market risk to which the subscribers are exposed with respect to the Exchangeable Bonds that they acquire (the "Delta Placement"). The placement price for the Shares sold in the Delta Placement shall be determined via an accelerated bookbuilding process that will be carried out by the Joint Bookrunners concurrently with the Equity Offering. Fresenius will not receive any proceeds, directly or indirectly, from any Shares sold pursuant to the Delta Placement.
This announcement is an advertisement and not a prospectus and not an offer of securities for sale in or into any jurisdiction, including the United States, Canada, Australia, Japan, South Africa or any jurisdiction in which offers or sales of the securities would be prohibited by applicable law. Neither this announcement nor anything contained herein shall form the basis of, or be relied upon in connection with, any offer or commitment whatsoever in any jurisdiction.
This announcement is not an offer to sell, or solicitation of an offer to buy, any securities in the United States. The securities described herein have not been, and will not be, registered under the U.S. Securities Act of 1933, as amended (the "Securities Act"), and may not be offered or sold in the United States absent registration or an exemption from, or in a transaction not subject to, the registration requirements of the Securities Act. There will be no public offering of the securities described herein in the United States or anywhere else and, if offered, any such securities will be offered and sold only (i) outside of the United States in "offshore transactions" in accordance with Regulation S of the Securities Act and/or (ii) in the United States to "qualified institutional buyers" (as defined in Rule 144A under the Securities Act) in transactions exempt from the registration requirements of the Securities Act.
This document and the offer when made, in member states of the European Economic Area ("EEA) (each a "Member State") and the United Kingdom, are only addressed to and directed at persons who are "qualified investors" as defined in the EU Prospectus Regulation or the UK Prospectus Regulation ("Qualified Investors"). Each person in a Member State or in the United Kingdom who initially acquires any securities described herein or to whom any offer of such securities may be made and, to the extent applicable, any funds on behalf of which such person is acquiring the Bonds that are located in a Member State or in the United Kingdom will be deemed to have represented, acknowledged and agreed that it is a Qualified Investor.
In addition, in the United Kingdom, this document is only being distributed to and is only directed at (i) persons who have professional experience in matters relating to investments falling within Article 19(5) of the Financial Services and Markets Act 2000 (Financial Promotion) Order 2005, as amended (the "Order"), (ii) high net worth entities falling withing Article 49(2) of the Order and (iii) persons at or to whom it can otherwise lawfully be distributed or directed (all such persons together being referred to as "relevant persons"). The securities are only available to, and any invitation, offer or agreement to subscribe, purchase or otherwise acquire such securities will be engaged in only with, relevant persons. Any person who is not a relevant person should not act or rely on this notification or any of its contents.
The information contained in this announcement is for background purposes only and does not purport to be full or complete. No reliance may be placed for any purpose on the information contained in this announcement or its accuracy or completeness. No prospectus will be prepared in connection with the offering of the securities referred to herein. The securities referred to herein may not be offered to the public in any jurisdiction in circumstances which would require the preparation or registration of any prospectus or offering document relating to the securities referred to herein in such jurisdiction.
This announcement may include statements that are, or may be deemed to be, "forward‐looking statements". These forward‐looking statements may be identified by the use of forward‐looking terminology, including the terms "believes", "estimates", "plans", "projects", "anticipates", "expects", "intends", "may", "will" or "should" or, in each case, their negative or other variations or comparable terminology, or by discussions of strategy, plans, objectives, goals, future events or intentions. Forward‐looking statements may and often do differ materially from actual results. Any forward‐looking statements reflect the Company's current view with respect to future events and are subject to risks relating to future events and other risks, uncertainties and assumptions relating to its business, results of operations, financial position, liquidity, prospects, growth or strategies. Forward‐looking statements speak only as of the date they are made.
The Company and its affiliates as well as the Joint Bookrunners, the Co-Lead Manager and their respective affiliates expressly disclaim any obligation or undertaking to update, review or revise any forward-looking statement contained in this announcement whether as a result of new information, future developments or otherwise.
Solely for the purposes of the product governance requirements contained within: (a) EU Directive 2014/65/EU on markets in financial instruments, as amended ("MiFID II"); (b) Articles 9 and 10 of Commission Delegated Directive (EU) 2017/593 supplementing MiFID II; (c) local implementing measures (together, the "MiFID II Product Governance Requirements"); and (d) the FCA Handbook Product Intervention and Product Governance Sourcebook (the "UK MiFIR Product Governance Rules"), and disclaiming all and any liability, whether arising in tort, contract or otherwise, which any "manufacturer" (for the purposes of the MiFID II Product Governance Requirements and UK MiFIR Product Governance Rules) may otherwise have with respect thereto, the Bonds have been subject to a product approval process, which has determined that: (i) the target market for the Bonds is eligible counterparties and professional clients only, each as defined in MiFID II and the UK MiFIR Product Governance Rules; and (ii) all channels for distribution of the Bonds to eligible counterparties and professional clients are appropriate. Any person subsequently offering, selling or recommending the Bonds (a "distributor") should take into consideration the manufacturer's target market assessment; however, a distributor subject to MiFID II or the UK MiFIR Product Governance Rulesis responsible for undertaking its own target market assessment in respect of the Bonds (by either adopting or refining the manufacturer's target market assessment) and determining appropriate distribution channels. The target market assessment is without prejudice to the requirements of any contractual or legal selling restrictions in relation to any offering of the Bonds and/or the underlying shares. For the avoidance of doubt, the target market assessment does not constitute: (a) an assessment of suitability or appropriateness for the purposes of MIFID II or the UK MiFIR Product Governance Rules; or (b) a recommendation to any investor or group of investors to invest in, or purchase, or take any action whatsoever with respect to the Bonds.
The Bonds are not intended to be offered, sold or otherwise made available to and should not be offered, sold or otherwise made available to any retail investor in the EEA or the United Kingdom (the "UK"). For these purposes, a "retail investor" means (a) in the EEA, a person who is one (or more) of: (i) a retail client as defined in point (11) of Article 4(1) of MIFID II; (ii) a customer within the meaning of Directive (EU) 2016/97 (as amended, the "Insurance Distribution Directive"), where that customer would not qualify as a professional client as defined in point (10) of article 4(1) of MIFID II, and (b) in the UK, a person who is one (or more) of (i) a retail client, within the meaning of Regulation (EU) no 2017/565 as it forms part of UK domestic law by virtue of the EUWA or (ii) a customer within the meaning of the provisions of the Financial Services and Markets Act 2000 of the UK (the "FSMA") and any rules or regulations made under the FSMA to implement Directive (EU) 2016/97, where that customer would not qualify as a professional client, as defined in point (8) of Article 2(1) of regulation (EU) No 600/2014 as it forms part of UK domestic law by virtue of the EUWA.
Consequently, no key information document required by Regulation (EU) No 1286/2014 (the "EU PRIIPs Regulation") or the EU PRIIPS Regulation as it forms part of UK domestic law by virtue of the EUWA (the "UK PRIIPS Regulation") for offering or selling the Bonds or otherwise making them available to retail investors in the EEA or the UK has been prepared and therefore offering or selling the Bonds or otherwise making them available to any retail investor in the EEA or the UK may be unlawful under the EU PRIIPs Regulation and/or the UK PRIIPS Regulation.
The Joint Bookrunners and the Co-Lead Manager are acting exclusively for the Company and no-one else in connection with the Combined Offering. They will not regard any other person as their respective clients in relation to the Combined Offering and will not be responsible to anyone other than the Company for providing the protections afforded to its clients, nor for providing advice in relation to the Combined Offering, the contents of this announcement or any transaction, arrangement or other matter referred to herein.
Any decision to purchase any of the securities described herein should only be made on the basis of an independent review by a prospective investor of the Company's publicly available information. Neither the Joint Bookrunners nor the Co-Lead Manager nor any of their respective affiliates nor any of its or their respective directors, officers, employees, advisers or agents accepts any liability arising from the use of, or make any representation as to the accuracy or completeness of, this announcement or the Company's publicly available information.
No reliance may or should be placed by any person for any purposes whatsoever on the information contained in this announcement or on its completeness, accuracy or fairness. The information in this announcement is subject to change.
Conference Call FY/2024 Presentation
FY/24: Upgraded outlook achieved, consistent financial performance with profitable growth.
Q4/2024: Continued growth and further deleveraging
1 Before special items
2 Organic growth rate adjusted for accounting effects related to Argentina hyperinflation.
3 Growth rate adjusted for Argentina hyperinflation.
4 Excluding Fresenius Medical Care
5 At average exchange rates for both net debt and EBITDA; pro forma closed acquisitions/divestitures, including lease liabilities, including Fresenius Medical Care dividend
Michael Sen, CEO of Fresenius: “Thanks to a tremendous team effort, Fresenius delivered outstanding results in 2024 with high-single-digit organic revenue growth and double-digit EBIT and EPS growth. Our growth vectors – Nutrition, MedTech and Biopharma – and consistent performance from Helios paced this strong development. On top of this operating success, we ended the year with a significant reduction in leverage, which is at the lowest level in seven years. The momentum for success will continue through 2025, as we move to the next phase of #FutureFresenius and take the company to the next level of performance. For 2025 we expect 4% to 6% in revenue growth and 3% to 7% in EBIT growth. We have also upgraded our ambition level of the Fresenius Financial Framework. This includes higher margin ambitions for Kabi, and for the Group a lower leverage corridor. We also want to pass on our improving financial strength to our shareholders. Thus, we want to recommend a dividend payment for the year of 1 Euro per share. As we move forward, we continue to focus on performance and delivery. Our mission to save and improve human lives is unwavering: Fresenius is Committed to Life."
Outlook for Fiscal Year 2025
Fresenius Group5: Organic revenue growth1,2 of 4% to 6%,
constant currency EBIT growth3 in the range of 3% to 7%
Fresenius Kabi1: Organic revenue growth2,3 in the mid- to high-single-digit percentage range; EBIT margin of 16.0% to 16.5%
Fresenius Helios4: Organic revenue growth2 in the mid-single-digit percentage range; EBIT margin3 around 10%
Assumptions to guidance: Guidance assumes current factors and known uncertainties, but it does not reflect potential extreme scenarios from a fast-moving geopolitical environment.
1 Before special items
2 Organic growth rate adjusted for accounting effects related to Argentina hyperinflation.
3 Growth rate adjusted for Argentina hyperinflation.
4 Excluding Fresenius Medical Care
5 2024 base: €21,526 million (revenue) and €2,489 million (EBIT)
Fresenius Financial Framework – Ambitions further raised
New dividend policy reflects capital allocation priorities
Fresenius’ new dividend policy is designed to ensure attractive shareholder returns while at the same time providing strategic flexibility. Going forward, Fresenius will pay out 30 to 40% of its Group core net income excluding Fresenius Medical Care and before special items as dividend. For fiscal year 2024, Fresenius will propose a dividend of €1.00 per share. The dividend proposal is a strong increase over the 2022 base and demonstrates Fresenius’ improving financial strength and its commitment to delivering shareholder value. For fiscal year 2023, Fresenius’ dividend payment was interrupted by legal restrictions due to the receipt of the energy relief payments at Helios in Germany.
1 2024 base: €8,414 million (revenue) and €1,319 million (EBIT)
2 Organic growth rate adjusted for accounting effects related to Argentina hyperinflation.
3 Before special items
4 2024 base: €12,739 million (revenue) and €1,288 million (EBIT)
5 At expected average exchange rates for both net debt and EBITDA; pro forma closed acquisitions/divestitures; excluding further potential acquisitions/divestitures; before special items; including lease liabilities, including Fresenius Medical Care dividend
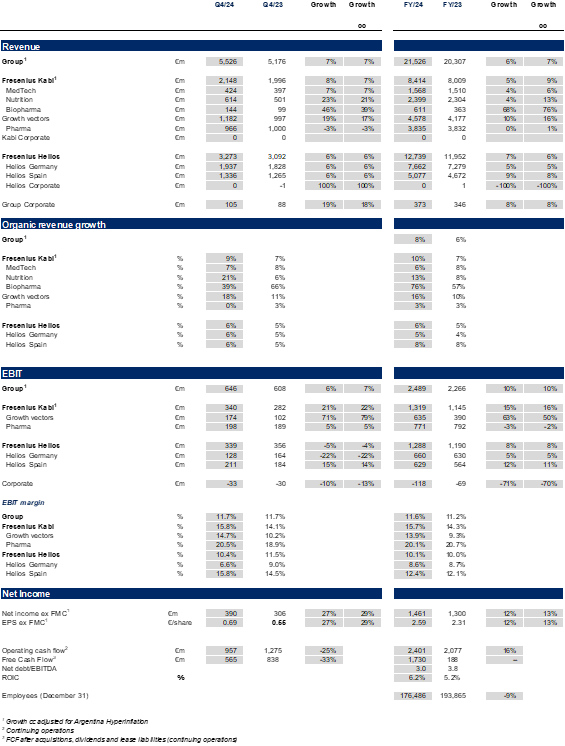
Fresenius Group – Business development FY/24
Fresenius closed fiscal year 2024 with a strong fourth quarter and achieved its twice-upgraded full-year guidance. The consistent positive delivery of Fresenius Kabi and the strong performance at Fresenius Helios, drove an 8%1 year-on-year Group organic revenue1 increase to €21.5 billion. Due to an improved operating business performance, Group EBIT before special items increased 10%3 in constant currency to €2.5 billion. Earnings per share2,4 rose by 13%3 in constant currency to €2.59.
End of 2024, the #FutureFresenius Revitalize phase has been successfully concluded, resulting in significant financial progress driven by a simpler Group structure, improved steering, an optimized portfolio and a refined operating model. In 2025, the focus will be on continued value creation by entering the Rejuvenate phase, which also aims to pursue platform-driven growth. In 2025 the emphasis will be on further debt reduction, delivering higher Kabi margins, drive Helios’ programme and fostering innovation.
A dedicated performance programme for Helios has been set up to increase efficiency and productivity, and to counteract the end of the energy relief funding. The programme is expected to contribute ~€100 million at EBIT level by 2025 at Helios Germany. Combined with further incremental growth of Helios in Germany and Spain, the Fresenius Helios EBIT margin is expected to be around 10% in FY/25. Contributions from the performance programme will be weighted to the second half of 2025, in particular, as some of the levers are process-related and will take time to deliver and realize benefits. Some of the performance measures are likely to materialize fully beyond 2025. This sets an excellent basis for further improving productivity within the 10 to 12% structural margin band in 2026 and beyond.
1 Organic growth rate adjusted for accounting effects related to Argentina hyperinflation.
2 Before special items
3 Growth rate adjusted for Argentina hyperinflation
4 Ex Fresenius Medical Care
Operating Companies – Business development FY/24 and Q4
Fresenius Kabi
In FY/24, Fresenius Kabi delivered consistent financial performance over the course of the year with excellent organic revenue growth of 10% above the top-end of the structural growth band and an impressive EBIT margin expansion of 140 bps to 15.7%.
Q4/24: Fresenius Kabi delivered a strong finish to the year
1 Organic growth rate adjusted for the accounting effects related to Argentina hyperinflation.
2 Before special items
Fresenius Helios
In FY 2024, Fresenius Helios delivered organic revenue growth of 6% driven by solid activity growth and favorable price developments in Germany and Spain. EBIT margin of 10.1%1 within the structural margin band ambition.
Q4/24: Fresenius Helios with strong EBIT development in Spain; end of energy relief payments weighing on Helios Germany
1 Before special items
Group figures Q4 & FY 2024
Conference call and Audio webcast
As part of the publication Fourth Quarter and Full Year 2024 results, a conference call will be held on February 26, 2025 at 1:30 p.m. CET (7:30 a.m. EST). All investors are cordially invited to follow the conference call in a live audio webcast at www.fresenius.com/investors. Following the call, a replay will be available on our website.
Contact for shareholders
Investor Relations
Telephone: + 49 61 72 6 08-24 87
Telefax: + 49 61 72 6 08-24 88
E-mail: ir-fre@fresenius.com
Note on the presentation of financial figures
This release contains forward-looking statements that are subject to various risks and uncertainties. Future results could differ materially from those described in these forward-looking statements due to certain factors, e.g. changes in business, economic and competitive conditions, regulatory reforms, results of clinical trials, foreign exchange rate fluctuations, uncertainties in litigation or investigative proceedings, the availability of financing and unforeseen impacts of international conflicts. Fresenius does not undertake any responsibility to update the forward-looking statements in this release.
An overview of key financial figures is available at the end of the release.
FY/24: Upgraded outlook achieved, consistent financial performance with profitable growth.
1 Before special items
2 Organic growth rate adjusted for accounting effects related to Argentina hyperinflation.
3 Growth rate adjusted for Argentina hyperinflation.
4 Excluding Fresenius Medical Care
5 At average exchange rates for both net debt and EBITDA; pro forma closed acquisitions/divestitures, including lease liabilities, including Fresenius Medical Care dividend
Q4/2024: Continued growth and further deleveraging
1 Before special items
2 Organic growth rate adjusted for accounting effects related to Argentina hyperinflation.
3 Growth rate adjusted for Argentina hyperinflation
4 Excluding Fresenius Medical Care
Michael Sen, CEO of Fresenius: “Thanks to a tremendous team effort, Fresenius delivered outstanding results in 2024 with high-single-digit organic revenue growth and double-digit EBIT and EPS growth. Our growth vectors – Nutrition, MedTech and Biopharma – and consistent performance from Helios paced this strong development. On top of this operating success, we ended the year with a significant reduction in leverage, which is at the lowest level in seven years.
The momentum for success will continue through 2025, as we move to the next phase of #FutureFresenius and take the company to the next level of performance. For 2025 we expect 4% to 6% in revenue growth and 3% to 7% in EBIT growth. We have also upgraded our ambition level of the Fresenius Financial Framework. This includes higher margin ambitions for Kabi, and for the Group a lower leverage corridor.
We also want to pass on our improving financial strength to our shareholders. Thus, we want to recommend a dividend payment for the year of 1 Euro per share.
As we move forward, we continue to focus on performance and delivery. Our mission to save and improve human lives is unwavering: Fresenius is Committed to Life."
Outlook for Fiscal Year 2025
Fresenius Group1: Organic revenue growth3,5 of 4% to 6%,
constant currency EBIT growth4 in the range of 3% to 7%
Fresenius Kabi2: Organic revenue growth3 in the mid- to high-single-digit percentage range; EBIT margin5 of 16.0% to 16.5%
Fresenius Helios6: Organic revenue growth5 in the mid-single-digit percentage range; EBIT margin5 around 10%
Assumptions to guidance: Guidance assumes current factors and known uncertainties, but it does not reflect potential extreme scenarios from a fast-moving geopolitical environment.
Fresenius Financial Framework – Ambitions further raised
New dividend policy reflects capital allocation priorities
Fresenius’ new dividend policy is designed to ensure attractive shareholder returns while at the same time providing strategic flexibility. Going forward, Fresenius will pay out 30 to 40% of its Group core net income excluding Fresenius Medical Care and before special items as dividend.
For fiscal year 2024, Fresenius wants to propose a dividend of €1.00 per share. The dividend proposal is a strong increase over the 2022 base and demonstrates Fresenius’ improving financial strength and its commitment to delivering shareholder value.
For fiscal year 2023, Fresenius’ dividend payment was interrupted by legal restrictions due to the receipt of the energy relief payments at Helios in Germany.
1 2024 base: €21,526 million (revenue) and €2,489 million (EBIT)
2 2024 base: €8,414 million (revenue) and €1,319 million (EBIT)
3 Organic growth rate adjusted for accounting effects related to Argentina hyperinflation
4 Growth rate adjusted for Argentina hyperinflation
5 Before special items
6 2024 base: €12,739 million (revenue) and €1,288 million (EBIT)
7 At expected average exchange rates for both net debt and EBITDA; pro forma closed
acquisitions/divestitures; excluding further potential acquisitions/divestitures; before
special items; including lease liabilities, including Fresenius Medical Care dividend
Fresenius Group – Business development FY/24
Fresenius closed fiscal year 2024 with a strong fourth quarter and achieved its twice-upgraded full-year guidance. The consistent positive delivery of Fresenius Kabi and the strong performance at Fresenius Helios drove an 8%1 year-on-year group organic revenue2 increase to €21.5 billion. Due to an improved operating business performance, Group EBIT before special items increased 10%3 in constant currency to €2.5 billion. Earnings per share2,4 rose by 13%3 in constant currency to €2.59.
End of 2024, the #FutureFresenius Revitalize phase has been successfully concluded, resulting in significant financial progress driven by a simpler Group structure, improved steering, an optimized portfolio and a refined operating model. In 2025, the focus will be on continued value creation by entering the Rejuvenate phase, which also aims to pursue platform-driven growth. In 2025 the emphasis will be on further debt reduction, delivering higher Kabi margins, drive Helios’ program and fostering innovation.
A dedicated performance programme for Helios has been set up to increase efficiency and productivity, and to counteract the end of the energy relief funding. The programme is expected to contribute ~€100 million at EBIT level by 2025 at Helios Germany. Combined with further incremental growth of Helios in Germany and Spain, the Fresenius Helios EBIT margin is expected to be around 10% in FY/25. Contributions from the performance programme will be weighted to the second half of 2025, in particular, as some of the levers are process-related and will take time to deliver and realize benefits. Some of the performance measures are likely to materialize fully beyond 2025. This sets an excellent basis for further improving productivity within the 10 to 12% structural margin band in 2026 and beyond.
1 Organic growth rate adjusted for accounting effects related to Argentina hyperinflation.
2 Before special items
3 Growth rate adjusted for Argentina hyperinflation
4 Ex Fresenius Medical Care
Operating Companies – Business development FY/24 and Q4
Fresenius Kabi
In FY/24, Fresenius Kabi delivered consistent financial performance over the course of the year with excellent organic revenue growth of 10%1 above the top-end of the structural growth band and an impressive EBIT margin2 expansion of 140 bps to 15.7%.
Q4/24: Fresenius Kabi delivered a strong finish to the year
1 Organic growth rate adjusted for the accounting effects related to Argentina hyperinflation.
2 Before special items
Fresenius Helios
In FY 2024, Fresenius Helios delivered organic revenue growth of 6% driven by solid activity growth and favorable price developments in Germany and Spain. EBIT margin1 of 10.1% within the structural margin band ambition.
Q4/24: Fresenius Helios with strong EBIT development in Spain; end of energy relief payments weighing on Helios Germany
1 Before special items
Financial figures and growth rates adjusted for the divestment of the fertility services group
Eugin and the hospital stake in Peru.
Group figures Q4 & FY 2024
Note on the presentation of financial figures
* * *
Conference call and Audio webcast
As part of the publication Fourth Quarter and Full Year 2024 results, a conference call will be held on February 26, 2025 at 1:30 p.m. CET (7:30 a.m. EST). All investors are cordially invited to follow the conference call in a live audio webcast at www.fresenius.com/investors. Following the call, a replay will be available on our website.
This release contains forward-looking statements that are subject to various risks and uncertainties. Future results could differ materially from those described in these forward-looking statements due to certain factors, e.g. changes in business, economic and competitive conditions, regulatory reforms, results of clinical trials, foreign exchange rate fluctuations, uncertainties in litigation or investigative proceedings, the availability of financing and unforeseen impacts of international conflicts. Fresenius does not undertake any responsibility to update the forward-looking statements in this release.
Fresenius SE & Co. KGaA
Registered Office: Bad Homburg, Germany / Commercial Register: Amtsgericht Bad Homburg, HRB 11852
Chairman of the Supervisory Board: Wolfgang Kirsch
General Partner: Fresenius Management SE
Registered Office: Bad Homburg, Germany / Commercial Register: Amtsgericht Bad Homburg, HRB 11673
Management Board: Michael Sen (Chairman), Pierluigi Antonelli, Sara Hennicken, Robert Möller, Dr. Michael Moser
Chairman of the Supervisory Board: Wolfgang Kirsch
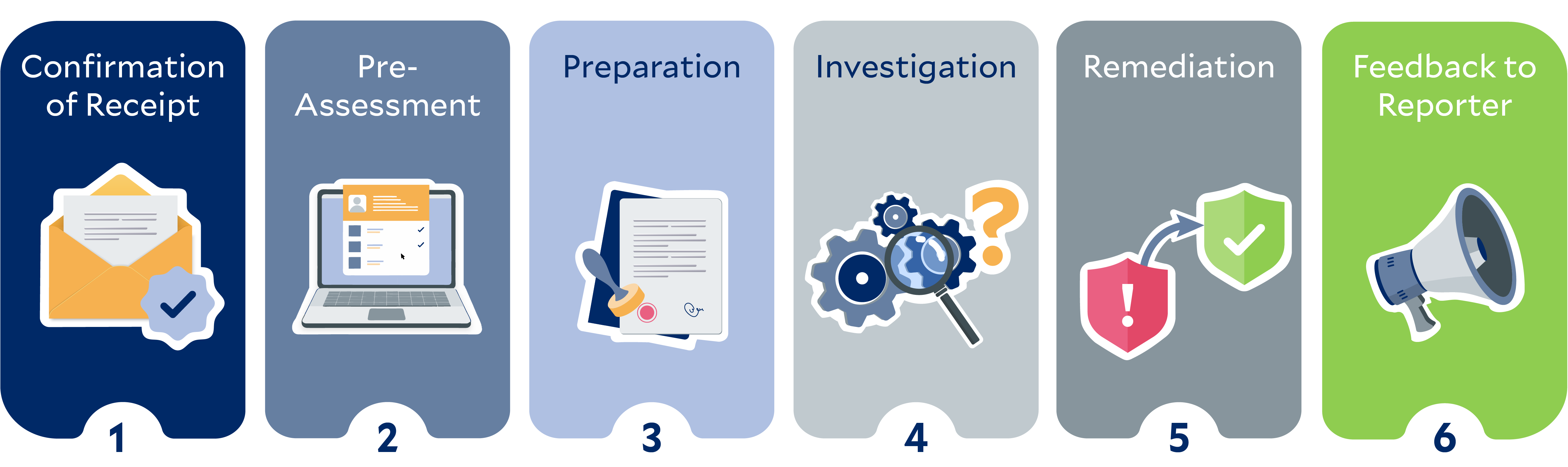
We value open communication and strive to create an environment in which patients, employees, members of local communities, business partners, or other potentially affected persons can report human rights violations or non-compliance with environmental regulations. To this end, we have set up whistleblower systems.
The following graphic illustrates the steps for processing reports, starting from the acknowledgment of receipt to the feedback provided to the person filing the report, using the example of two fictional protagonists: Kate and Aditi.*
*The persons and stories depicted in this image are purely fictional. Any resemblance to actual persons is purely coincidental and unintentional.
Additional information on our grievance mechanisms and the protection of whistleblowers can be found in the process descriptions of the respective companies:
Fresenius SE:
https://www.fresenius.com/grievance-mechanism
Fresenius Kabi:
https://www.fresenius-kabi.com/responsibility/business-ethics
Fresenius Helios:
https://www.helios-gesundheit.de/menschenrechte
Quirónsalud:
https://www.quironsalud.com/en/group/whistleblowing-channel
Have you ever had the feeling that something isn’t quite right? Perhaps you noticed something that shouldn’t be the way it is, or you yourself were affected by such a situation. That’s when it’s time to report a grievance.
Watch the video to find out how to report a grievance, to learn why reporting grievances is so important, and to see what happens to a report after you submit it.
The metrics describe the reports received through our reporting systems in the reporting year that were related to human rights – broken down into those affected in our own operations and those in our value chain. Of the 28 reports received, 4 were violations of working hours and rest breaks, remuneration and occupational health and safety.
No report was related to a severe human rights violation in the upstream or downstream value chain or in our own operations.1
| Own operations | Value chain | |
|---|---|---|
Reports received with human rights relevance | 25 | 3 |
Of which are violations | 4 | - |
Of which are severe human rights violations | - | - |
1Severe human rights violation include incidents of forced labor, incidents of human trafficking, incidents of child labor as well incidents involving a large number of people or affecting a large area. This categorization is based, for example, on the definitions of the Corporate Sustainability Reporting Directive (CSRD).
The Group-wide Sustainability Report 2024 (CSRD Report) contains detailed information on the substantiated cases and the remedial and preventive measures implemented in the chapters “Own workforce” and “Workers in the value chain”.
At Fresenius, we attach great importance to taking appropriate account of the interests of whistleblowers and those of potentially affected persons in the processing of reports. We achieve this through a transparent, fair and comprehensive examination of all incoming complaints by specially trained staff.
Our aim is to ensure that the concerns of those affected are taken seriously and processed in accordance with the applicable legal and internal company requirements. In doing so, we ensure that all relevant information and perspectives are included in the decision-making process to find a balanced and appropriate solution.
Through feedback from those affected, we analyze how our process is accepted in practice and where there is potential for optimization. This input is incorporated into the further development of our processes to ensure that the interests of those affected are taken into account even better in future.
The results of our risk analysis and the findings on potential target groups of our grievance and whistleblower channels are incorporated into the further development of our grievance mechanism and the processing of respective reports. Based on our findings, we review the effectiveness of the procedure on an annual basis, or more frequently if required. Where necessary, we make appropriate adjustments and changes with regard to accessibility and process.
Human Rights at Fresenius
Our Human Rights ProgramContact
Fresenius SE & Co. KGaA
Else-Kröner-Str. 1
61352 Bad Homburg
Germany
humanrights@fresenius.com
Human Rights Program
Our Human Rights Program Group-wide Governance & Responsibilities Risk Assessment & Impact Preventive & Remedial ActionYou want to file a report?
Reports on possible human rights violations or other types of compliance violations can be reported around the clock, either anonymously or by name, via our whistleblower system*:
Whistleblower system
https://freseniusgroup.ethicspoint.com
By telephone*
+49 (0) 800 181 1338
By mail
Fresenius SE & Co. KGgaA Business Integrity
Else-Kröner-Str. 1
61352 Bad Homburg v.d.H. Deutschland
By e-mail:
humanrights@fresenius.com
* The prices of your mobile or landline contract apply.
The reporting on the respect for human rights for the year 2024 and following can be found in our Sustainability Report (CSRD Report). Further information on Fresenius' human rights program is available here: fresenius.com/en/human-rights
To prevent, eliminate, or minimize human rights risks, both the Group and each business segment take appropriate preventive measures tailored to the individual case in our own business and in the value chain. In cases where our business activities have caused or contributed to human rights violations, we take appropriate and effective case-specific remedial action.
The following overview provides a non-exhaustive summary of potential standard preventive measures for Fresenius’s own operations and / or the value chain.
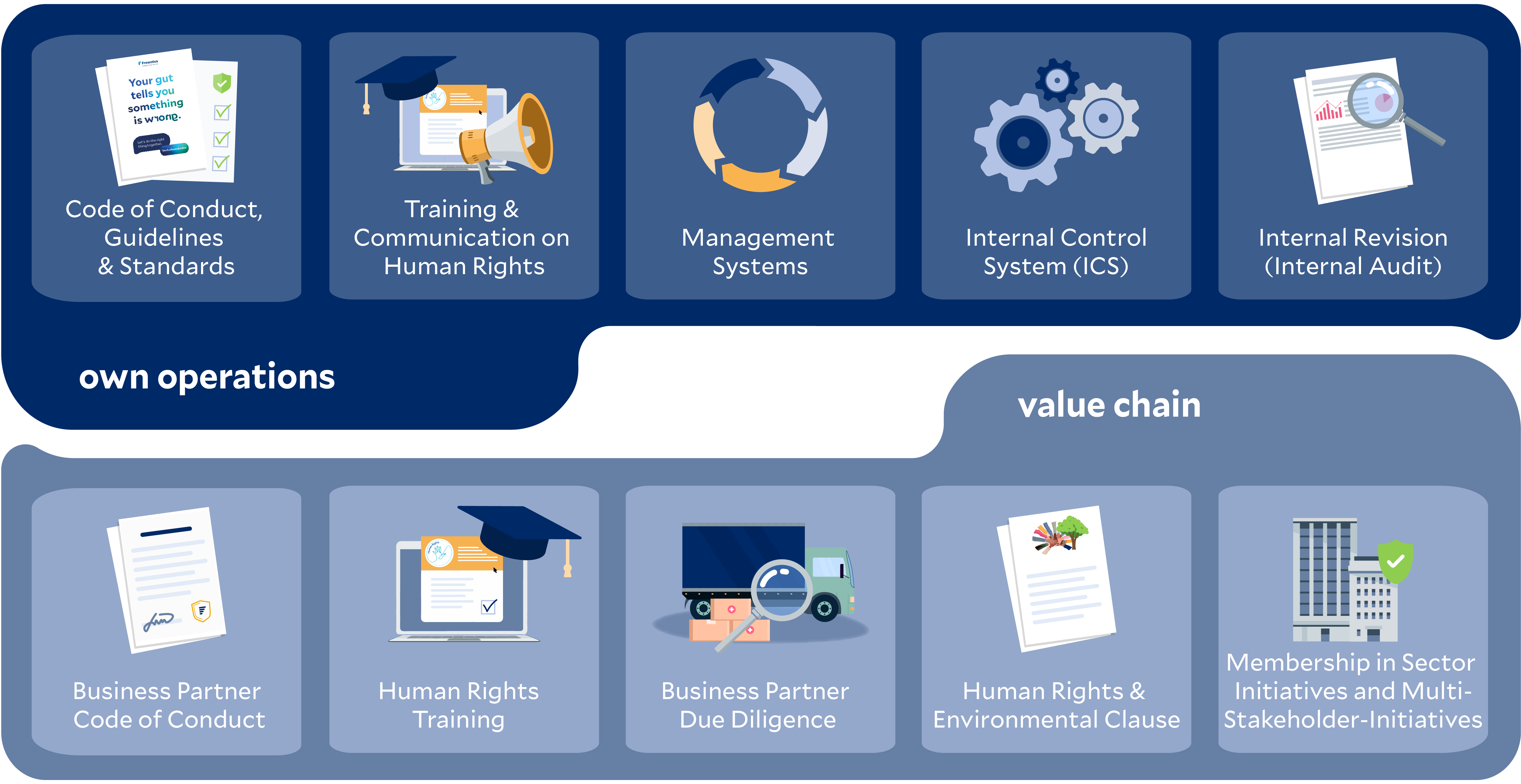
Standard preventive measures within our own operations include, among others, a binding Code of Conduct for our employees, guidelines on social and labor standards, as well as management systems for occupational health and safety.
In addition to training on human rights and communication measures related to our Human Rights Statement, our Internal Control System (ICS) and regular internal audits strengthen the implementation and monitoring of our human rights due diligence obligations.
Our measures within the value chain include, among other things, a Code of Conduct for business partners as well as risk-based training on human rights requirements, including information on the grievance mechanism.
These are complemented by risk-based assessments and ongoing monitoring of our business partners. Human rights and environmental clauses in contracts, along with our memberships in industry and multi-stakeholder initiatives, further support the implementation of our due diligence obligations throughout the value chain. In addition, within the scope of our influence, we develop specific prevention measures tailored to the results of regular and event-driven risk analyses and document these accordingly.
Further information on specific preventive measures can be found under Risk Analysis and Impact as well as in the 2024 CSRD report in the chapters “Own workforce” and “Workers in the value chain”.
What are human rights? How can I report a possible violation? And what does the term human rights due diligence mean? Our employees and business partners shall know the answers to these questions if we are to put our commitment to human rights into practice.
For this reason, and in addition to target-group specific training offerings on individual human rights topics, we have developed a dedicated global human rights training course – together with colleagues from different parts of our organization. This training has been gradually introduced for our employees since 2025 and also serves as a supporting measure in our collaboration with our suppliers. Which suppliers should take part in the training depends on their respective risk profiles. In this way, we want to actively contribute to the further development of our corporate culture and create a common understanding of due diligence obligations in our value chain.
The aim of any remedial action is to end or minimize and, if possible, reverse the human rights or environmental violation. To measure effectiveness, we review the implementation of the measures at a case-specific interval. If necessary, we initiate further measures. A process is only considered closed when all remediation measures have been fully implemented.
To address negative impacts on rightsholders, we have developed a toolbox to provide practical support for human rights specific remediation measures. This is aimed at colleagues involved in investigating human rights and environmental violations affecting employees of Fresenius as well as workers in the value chain and consists of various components. These include general guidance on remediation in accordance with the LkSG and international human-rights-related standards and principles. It also includes guidance on dealing with specific human rights violations and a handout for evaluating the effectiveness of remediation.
We continuously review the effectiveness of preventive and corrective measures after implementation. This evaluation also incorporates new insights, such as those from our risk analysis and feedback from the affected stakeholder groups. If required, we initiate targeted adjustments to further enhance the effectiveness of the measures.

Human Rights at Fresenius
Our Human Rights ProgramContact
Fresenius SE & Co. KGaA
Else-Kröner-Str. 1
61352 Bad Homburg
Germany
humanrights@fresenius.com
Human Rights Program
Our Human Rights Program Group-wide Governance & Responsibilities Risk Assessment & Impact Grievance Procedure & HandlingWhistleblowing system
Reports on possible human rights or other types of compliance violations can be reported around the clock, either anonymously or by name, via our whistleblower system*:
Phone number: +49 (0) 800 181 1338*
https://freseniusgroup.ethicspoint.com
More infos about our grievance mechanism
*The prices of your mobile or landline contract apply
The reporting on the respect for human rights for the year 2024 and following can be found in our Sustainability Report (CSRD Report). Further information on Fresenius' human rights program is available here: fresenius.com/en/human-rights
Human rights risks can change over time. We therefore conduct annual and event-related risk analyses. In the 2024 reporting period, this was done in the third and fourth quarters. An event-related risk analysis was not conducted.
Identifying and assessing human rights risks in our own company and in our value chain is a comprehensive process that consists of risk identification, risk analysis, and risk assessment. We follow a risk-based approach that can be divided into three phases and is explained below with the help of the fictional protagonist, John.*
*The persons and stories depicted in these images are purely fictional. Any resemblance to actual persons is purely coincidental and unintentional.
To weight and prioritize risks, we introduced a comprehensive methodology for assessing their impact and likelihood of occurring. Using this method, the risks are plotted on a matrix (4x4).
The evaluated impact on those affected ranges from “low” to “severe”. It is assessed using four evaluation criteria: scope, scale, possibility for remedial action, and company involvement.
Probability of occurrence ranges from “unlikely” to “almost certain”. It is assessed using three different evaluation criteria: process evaluation, evaluation of similar cases which have already arisen, and context factors which could increase the likelihood of the risks materializing.
We carry out a regular risk analysis of human rights topics for both our own businesses and our supply chains in accordance with the specific requirements of applicable international and national laws and regulations. As part of this risk analysis, we identify areas that we consider to have high priority due to the potential severity of possible violations and due to our ability to influence them.
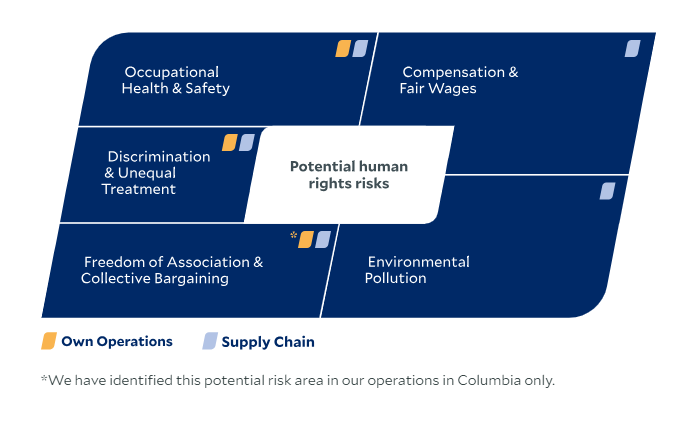
The risk analysis for the 2024 reporting year confirmed the following existing prioritized risk areas for our own operations and revealed additional potential risks:
As a healthcare Group, we not only bear responsibility for the well-being of our patients, but also for the health and safety of our employees. We implemented a Group Policy on Social and Labor Standards. The guideline describes our global social and labor law minimum standards. We expect our employees and managers in all business segments of the Fresenius Group to comply with this guideline without exception. Lower standards are not acceptable. Should laws or practices in countries where we operate restrict or contradict the standards set out in this policy, we will apply the policy to the extent permitted by local laws . Moreover, the Fresenius Code of Conduct stipulates that we take the necessary measures to protect our employees and prevent work-related accidents and illnesses.
Creating a safe and healthy working environment is a priority for us. When it comes to health protection, prevention is our basic principle: We therefore provide our employees with comprehensive programs to promote their health and prevent work-related illnesses. The return of employees after an illness is regulated, for example, by the company integration management system.
We have introduced numerous management systems and measures throughout the Group and adapted them to the business models of the business segments. They focus on occupational health and safety in the production area as well as occupational health management for employees in healthcare facilities or in administration.
All locations are also subject to the respective local regulations and laws. Compliance with these regulations is ensured at local level. In addition to statutory regulations, internal guidelines and directives such as management manuals and standard operating procedures also play a significant role in occupational health and safety. In addition to the Group-wide Fresenius Code of Conduct, the business segments have their own guidelines that regulate occupational health and safety, e.g. the Clinical Code of Conduct for the rehabilitation and nursing units and medical personnel in the healthcare services market segment.
The internal requirements are supplemented by corresponding internationally recognized standards for management systems such as ISO 45001 at some locations as well as other certifications in accordance with ISO or national standards. The overarching aim of the ISO 45001 management system is to continuously improve occupational health and safety management, align it with internationally recognized methods and ensure the effectiveness of existing procedures and systems. To drive this forward, we are consistently expanding the number of entities certified to this standard.
Further Information can be found in the 2024 CSRD report from page 216.
To minimize potential human rights risks connected to our business activities, we have taken further preventive and mitigating measures. These include, for example, training that we offer to our workforce. The Fresenius Group, for instance, conducts regular occupational health and safety training to prevent incidents in its fields of operation.
To sustainably promote tolerance and appreciation within our teams in the long-term and to mitigate the risk of discrimination, it furthermore is not only necessary to have a corresponding culture that is exemplified by the management bodies; employees also receive training and further education on the topic of diversity.
The elimination of discrimination is both a component of our Group-wide compliance programs and a key element of our Human Rights Program. These concepts are supplemented by suitable controls, process documentation, training concepts, awareness-raising measures, and the use of whistleblower systems. In this way, we want to ensure that discrimination, including harassment, is prevented, contained, or combated in our operational business if we become aware of violations, risks, or impacts.
Another central element is the successive roll out of a company-wide human rights training for our employees. The training aims at educating our employees on this important topic – not only about their personal human rights, but also about the contribution that everyone can make in their daily work. It further imparts knowledge about individual rights and how to deal with possible human rights violations.
In addition, we have implemented individual and local measures to provide our employees with the best possible protection within our sphere of influence. For instance, at high-risk locations, we deploy more security staff than is typically found in hospitals to better protect our employees and patients around the clock. Furthermore, a monthly committee with participants from local management and employees has been established to strengthen the dialogue.
Further details on preventive and remedial measures, as well as on appropriateness and effectiveness, are available here.
The risk analysis for the 2024 reporting year confirmed the following existing prioritized risk areas for our value chain and revealed additional potential risks:
To counter the potentially negative effects, we have initiated and implemented further preventive measures in addition to existing ones. With the risk-based implementation of human rights and environmental clauses in contracts, we also agree with suppliers on specific requirements for cooperation and information obligations in the event of human rights violations.
Our Code of Conduct for Business Partners also sets out fundamental expectations regarding respect for human rights. To monitor compliance with these principles and use the results to provide industry-wide support, Fresenius Kabi, for example, prepared to join the Pharmaceutical Supply Chain Initiative (PSCI) as an associate member in the reporting year (2024). In the future, the company will participate in the industry-wide audit pooling and thus contribute to greater transparency regarding working conditions and – where necessary – corresponding corrective or remedial measures in the pharmaceutical supply chain.

Marco Kraemer, Director Supplier Quality Management & Human Rights Function, Fresenius Kabi
To further increase transparency in our upstream and downstream processes, we also plan to further expand the existing descriptions and visualizations of our value chains and carry out focus risk analyses on this basis. Further details on preventive and remedial measures, as well as on appropriateness and effectiveness, are available here.

Human Rights at Fresenius
Our Human Rights ProgramContact
Fresenius SE & Co. KGaA
Else-Kröner-Str. 1
61352 Bad Homburg
Germany
humanrights@fresenius.com
Human Rights Program
Our Human Rights Program Group-wide Governance & Responsibilities Preventive & Remedial Action Grievance Procedure & HandlingWhistleblowing system
Reports on possible human rights or other types of compliance violations can be reported around the clock, either anonymously or by name, via our whistleblower system*:
Phone number: +49 (0) 800 181 1338*
https://freseniusgroup.ethicspoint.com
More infos about our grievance mechanism
*The prices of your mobile or landline contract apply
The reporting on the respect for human rights for the year 2024 and following can be found in our Sustainability Report (CSRD Report). Further information on Fresenius' human rights program is available here: fresenius.com/en/human-rights