Fresenius Medical Care, the world’s leading provider of dialysis services and products, plans to invest EUR 60 million in its independent affiliate Unicyte AG in a Series A financing round. Unicyte, a leading regenerative medicine company with translational programs in the field of kidney disorders and other diseases, will primarily use the capital to start clinical trials of its first product candidates in 2020 and to establish the required manufacturing processes.
Over the last four years, Unicyte has created a unique set of proprietary technology platforms of human liver stem cells (HLSCs), HLSC-derived islets and nano-Extracellular Vesicles (nEVs). nEVs are stem cell-derived particles that support communication between cells.
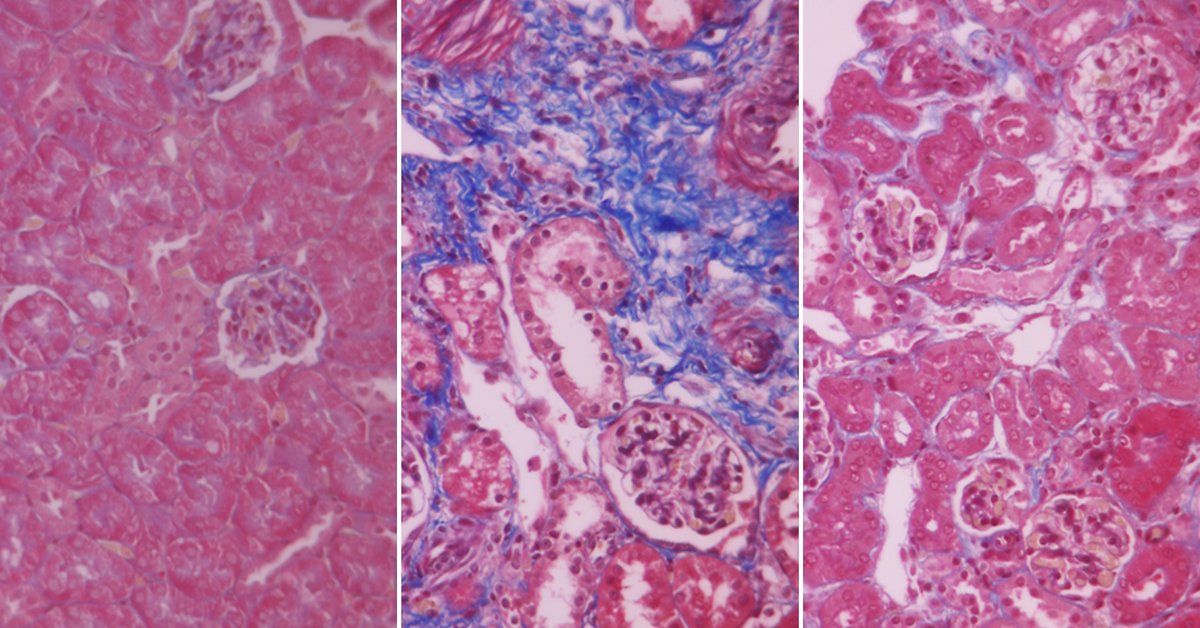
Unicyte succeeded in confirming the disease-modifying potential of its nEVs in various preclinical models of chronic kidney disorders. Combined results of these studies demonstrate nEVs’ efficacy and underlying mechanism of action in preventing renal fibrosis and its subsequent progression to end-stage renal disease.
“Regenerative medicine could provide highly innovative therapies to chronic kidney disease patients and is becoming increasingly important for our industry. Driving regenerative therapies not only addresses the U.S. administration’s initiative to improve the prevention of end-stage renal disease, but can help us to significantly slow the progression of kidney disease and make the most innovative therapies available to our patients,” said Rice Powell, CEO of Fresenius Medical Care.
Dr. Olaf Schermeier, Fresenius Medical Care’s CEO for Global Research and Development, said: “This continued investment in Unicyte shows our commitment to developing the best treatment options for our patients across the renal care continuum. At the same time, it puts Fresenius Medical Care at the forefront of innovation in the field of regenerative renal stem cell therapy.”
“Based on our preclinical results, the commitment of Fresenius Medical Care is a strong driver for Unicyte’s next milestone, the start of clinical development with the required organizational structure. It will also provide a solid data package for our non-kidney product candidates to seek strategic partnerships as the next step,” said Florian Jehle, CEO of Unicyte.
This release contains forward-looking statements that are subject to various risks and uncertainties. Actual results could differ materially from those described in these forward-looking statements due to certain factors, including changes in business, economic and competitive conditions, regulatory reforms, foreign exchange rate fluctuations, uncertainties in litigation or investigative proceedings, and the availability of financing. These and other risks and uncertainties are detailed in Fresenius Medical Care AG & Co. KGaA's reports filed with the U.S. Securities and Exchange Commission. Fresenius Medical Care AG & Co. KGaA does not undertake any responsibility to update the forward-looking statements in this release.
September 05, 2019
Brussels, Belgium
Société Générale – European Angle Conference
September 09, 2019
New York, USA
Morgan Stanley – 17th Annual Global Healthcare Conference
September 04, 2019
London, UK
Goldman Sachs – 16th Annual European Medtech and Healthcare Services Conference
Quirónsalud, Spain’s largest private hospital group and part of Fresenius Helios, has acquired Clínica Las Vegas and Clínica del Prado and further expanded its presence in the attractive private hospital market in Colombia. Clínica Las Vegas and Clínica del Prado are two centrally located hospitals in Medellín, a major city of 2.5 million people. The two facilities have a total of about 300 beds. The total investment for both hospitals is about €50 million.
Following Quirónsalud's entry into Peru in 2017 and the acquisition of Clínica Medellín this year, this is another step in strengthening the company’s presence in Latin America’s growing and consolidating hospital markets. Fresenius Helios expects both transactions to close in Q4 2019, pending anti-trust and regulatory clearance by the Colombian authorities.
This release contains forward-looking statements that are subject to various risks and uncertainties. Future results could differ materially from those described in these forward-looking statements due to certain factors, e.g. changes in business, economic and competitive conditions, regulatory reforms, results of clinical trials, foreign exchange rate fluctuations, uncertainties in litigation or investigative proceedings, and the availability of financing. Fresenius does not undertake any responsibility to update the forward-looking statements in this release.
Phenylephrine Hydrochloride Injection is the latest addition to the company´s growing anesthesia and analgesia portfolio.
Quirónsalud, Spain’s largest private hospital group and part of Fresenius Helios, has acquired Clínica Las Vegas and Clínica del Prado and further expanded its presence in the attractive private hospital market in Colombia. Clínica Las Vegas and Clínica del Prado are two centrally located hospitals in Medellín, a major city of 2.5 million people. The two facilities have a total of about 300 beds. The total investment for both hospitals is about €50 million.
Following Quirónsalud's entry into Peru in 2017 and the acquisition of Clínica Medellín this year, this is another step in strengthening the company’s presence in Latin America’s growing and consolidating hospital markets. Fresenius Helios expects both transactions to close in Q4 2019, pending anti-trust and regulatory clearance by the Colombian authorities.
This release contains forward-looking statements that are subject to various risks and uncertainties. Future results could differ materially from those described in these forward-looking statements due to certain factors, e.g. changes in business, economic and competitive conditions, regulatory reforms, results of clinical trials, foreign exchange rate fluctuations, uncertainties in litigation or investigative proceedings, and the availability of financing. Fresenius does not undertake any responsibility to update the forward-looking statements in this release.

Fresenius Medical Care, the world’s leading provider of dialysis products and services, has appointed Helen Giza (51) as Chief Financial Officer as of November 1, 2019. She will succeed Mike Brosnan who announced his retirement from the Company earlier this year after serving as CFO since January 2010.
Helen Giza has been Chief Integration and Divestiture Management Officer at Takeda Pharmaceuticals since 2018. Before joining the Takeda Corporate Executive Team, she served as Chief Financial Officer of Takeda’s U.S. business unit since 2008. Prior to that she held a number of key international finance and controlling positions, amongst others at TAP Pharmaceuticals and Abbott Laboratories. Helen Giza is a U.K. Chartered Certified Accountant and holds a Master of Business Administration from the Kellogg School of Management at Northwestern University in Evanston, Illinois, USA.
Stephan Sturm, Chairman of the Supervisory Board of Fresenius Medical Care Management AG, said: “Helen Giza is a very skilled financial executive with extensive management experience in the healthcare industry. She will be a great addition to our team and we are very pleased to welcome her to Fresenius Medical Care’s management board.”
Rice Powell, Chief Executive Officer of Fresenius Medical Care and Chairman of the Management Board, said: “We look forward to welcoming Helen to our team. Along with her international financial expertise, Helen brings great experience in the area of acquisitions and successful integration within the healthcare sector.”
Helen Giza said: “I am excited to be joining Fresenius Medical Care, the market leader in dialysis. This new role is a wonderful opportunity to be part of Fresenius Medical Care's continued success.”
This release contains forward-looking statements that are subject to various risks and uncertainties. Actual results could differ materially from those described in these forward-looking statements due to certain factors, including changes in business, economic and competitive conditions, regulatory reforms, foreign exchange rate fluctuations, uncertainties in litigation or investigative proceedings, and the availability of financing. These and other risks and uncertainties are detailed in Fresenius Medical Care AG & Co. KGaA's reports filed with the U.S. Securities and Exchange Commission. Fresenius Medical Care AG & Co. KGaA does not undertake any responsibility to update the forward-looking statements in this release.
September 24, 2019
Munich, Germany
Berenberg and Goldman Sachs – 8th German Corporate Conference
September 24 – 25, 2019





