Intensive Care Unit 1
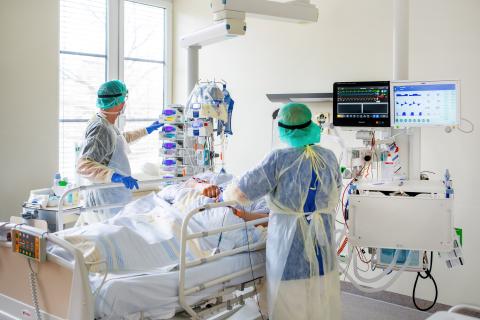
Treatment of a COVID-19 patient in the intensive care unit at Fresenius Helios.
Share
Services

Melanie Schmitt of Fresenius Medical Care in Schweinfurt is Germany’s best apprentice in the category “Electronics Technician for Information and Systems Technology.” This annual award from the Association of German Chambers of Industry and Commerce was based on her outstanding examination results at the end of a three-and-a-half-year training period. Schmitt, 20, was also honored for the best vocational qualification in the State of Bavaria and the Main-Franconia region.
Between September 2016 and February 2020, Schmitt trained in various departments in production and in research and development at Fresenius Medical Care’s facility in Schweinfurt. She learned to develop and program electronic switches and other hardware and software components. The Schweinfurt resident is now working in the software and systems verification department of the Global Research and Development division.
“If I do something, then I like to do it right,” Schmitt said, explaining her outstanding results to the small group gathered for the award presentation at the Schweinfurt plant. An annual ceremony is normally held in Berlin to honor all the apprentice winners from across Germany, but it had to be canceled this year due to the pandemic.
“As the local chamber, state and federal winner, Melanie Schmitt accomplished a gigantic feat,” said Jürgen Bode, Deputy Director of the Würzburg-Schweinfurt Chamber of Industry and Commerce, who presented the award. “This required good cooperation between the apprentice and the company providing the training. My thanks to Fresenius Medical Care, who have accomplished this superbly.”
Christian Hepp, head of industrial training at Fresenius Medical Care in Schweinfurt, said: “Quality in training is very important to us. We are really delighted, along with Melanie Schmitt, about this special award. And we are honored that she has decided on a future with us.”
During industrial and commercial training at Fresenius Medical Care in Schweinfurt, apprentices, trainees and students in dual-study programs work on various projects together with engineers and other experts. Assignments have a specific practical application, and there are few purely academic exercises.
Opened in 1979, the Schweinfurt facility is Fresenius Medical Care’s most important research and production facility for dialysis machines and other medical devices. The company currently has more than 1,300 employees at the site, about a third of them in research and development.
Icatibant injection, an FDA-approved and cost-effective alternative to treat acute attacks of hereditary angioedema in adults, expands the company’s generic drug portfolio. Icatibant Injection is supplied as a prefilled, single-use syringe that is self-administered subcutaneously at the patient’s home or doctor’s office. In conjunction with launching its first specialty IV drug in the U.S., Fresenius Kabi is also launching KabiCare in the U.S., a comprehensive patient support program with case managers, co-pay assistance programs, injection training, and a patient assistance program.

Treatment of a COVID-19 patient in the intensive care unit at Fresenius Helios.
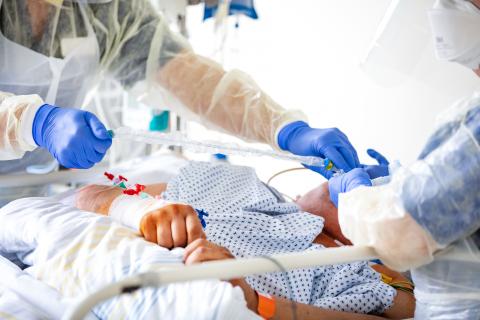
Artificial respiration of a COVID-19 patient in an intensive care unit at Fresenius Helios.
Continuous autotransfusion with a CATSmart system from Fresenius Kabi.
Fresenius Medical Care, the world’s leading provider of products and services for people with chronic kidney failure, has been recognized for the 11th time as a sustainability leader with inclusion in the Dow Jones Sustainability Index (DJSI Europe). The DJSI Europe index represents the top 20 percent of the largest 600 European companies in the S&P Global BMI, based on the international investment company S&P Global’s analysis of their economic, environmental and social performance.

May 21, 2021 - 10:00 am
Bad Homburg, Germany