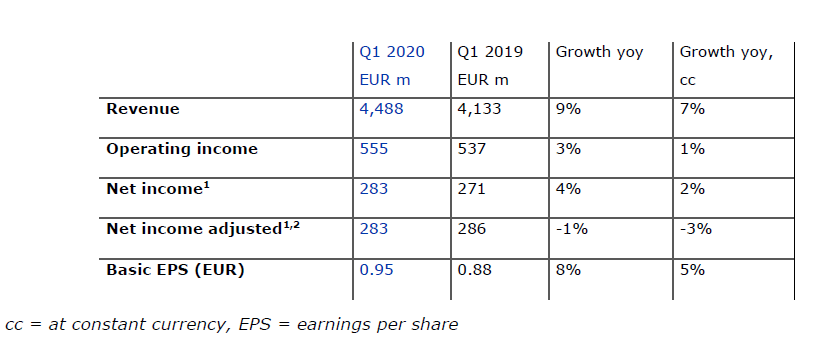
- 9% revenue increase supported by growth in all regions
- Positive earnings growth despite negative impact from COVID-19 pandemic
- Solid cash-flow development
- Financial targets for FY2020 confirmed
Rice Powell, Chief Executive Officer of Fresenius Medical Care, said: “In these unprecedented times, it is our first and foremost priority to maintain the continuity and high quality of care. For months now, our employees have been working tirelessly to ensure that our patients receive their life-saving dialysis treatments. I cannot thank them enough. We appreciate the financial commitment that the U.S. administration has given in April to support healthcare providers. The strong revenue growth in the first quarter shows that the underlying business development remains intact and that our business model is resilient. In a global pandemic that is redefining priorities in other areas of the healthcare system, dialysis remains essential for millions of patients worldwide.”
Fresenius Medical Care’s contribution in the fight against COVID-19
In order to ensure the continuity and high quality of care for dialysis patients during the COVID-19 pandemic and support its employees around the globe, Fresenius Medical Care has taken wide-ranging measures at a very early stage. The focus is on reducing the risk of infection in dialysis clinics for patients and employees.
Despite countries’ lockdown efforts to contain the COVID-19 pandemic, Fresenius Medical Care did not experience any major disruptions in its manufacturing facilities. As of today, all manufacturing plants worldwide are in operation and supply chains remain intact.
Costs in respect to the COVID-19 pandemic have been incurred for additional measures, like personal protective equipment, dedicated capacities for isolated treatments, additional personnel expense, patient transportation as well as increased distribution logistic costs.
Under the CARES Act (Coronavirus Aid, Relief, and Economic Security Act), the U.S. government initiated significant financial support for the health sector. This is intended, for example, to compensate for the increased costs for healthcare providers as a result of the COVID-19 pandemic and the corresponding protective measures. While Fresenius Medical Care had to adsorb a sizable negative impact in Q1, there is no benefit from the CARES Act included in the reported results.
In the U.S., Fresenius Medical Care is cooperating with other dialysis providers, to create isolation clinics and dedicated shifts for patients who are or may be infected with COVID-19. A critical aim of this collaboration is to keep dialysis patients out of the hospital whenever possible, freeing up limited hospital resources. In doing so, Fresenius Medical Care not only fulfils its responsibility towards patients, employees and families, but also makes an important contribution to the healthcare system and society as a whole.
Key figures (IFRS)
2020 targets confirmed: mid to high single digit growth rates
On the basis of the guidance given in February, which excludes the impact from the COVID-19 pandemic, Fresenius Medical Care expects both revenue and net income to grow at a mid to high single digit rate in 2020. These targets are in constant currency, exclude special items3 and are based on the adjusted results 2019 including the effects of the operations of the NxStage acquisition and the IFRS 16 implementation.
Patients, Clinics and Employees
As of March 31, 2020, Fresenius Medical Care treated 348,703 patients in 4,002 dialysis clinics worldwide. At the end of the first quarter, the Company had 121,403 employees (full-time equivalents) worldwide, compared to 118,308 employees as of March 31, 2019.
Strong revenue growth in the first quarter
Revenue increased by 9% to EUR 4,488 million (+7% at constant currency), with organic growth of 4%. Health Care Services revenue rose by 8% to EUR 3,595 million (+7% at constant currency), driven by growth in same market treatments, contributions from acquisitions and an increase in dialysis days. Health Care Products revenue grew by 10% and amounted to EUR 893 million (+9% at constant currency). This increase was mainly due to higher sales of products for acute care treatments, renal pharmaceuticals and bloodlines partially offset by lower sales of dialysis machines.
Operating income increased by 3% to EUR 555 million (+1% at constant currency), mainly driven by a favorable impact from higher treatment volume and lower costs for pharmaceuticals. The operating income margin amounted to 12.4% (Q1 2019: 13.0%). The decrease in margin was largely due to the unfavorable COVID-19 pandemic effect and the prior year reduction of a contingent consideration liability related to Xenios.
Despite the negative impact from the COVID-19 pandemic net income1 grew by 4% to EUR 283 million (+2% at constant currency) and declined on an adjusted basis by only 1% (-3% at constant currency). On the defined basis of the 2020 targets - excluding the negative impact from the COVID-19 pandemic - net income growth in the first quarter is at the top end of the target range for 2020.
Basic earnings per share (EPS) increased by 8% to EUR 0.95 (+5% at constant currency) driven by the earnings effects described above coupled with a decrease in the average weighted shares outstanding.
Solid cash-flow development
Fresenius Medical Care generated EUR 584 million of operating cash flow (Q1 2019: EUR 76 million) resulting in a margin of 13.0% (Q1 2019: 1.8%). The increase was largely driven by working capital improvement, including a positive effect from cash collections, timing of payments and change in year over year inventory levels.
Free cash flow (net cash used in operating activities, after capital expenditures, before acquisitions and investments) amounted to EUR 304 million (Q1 2019: EUR -123 million), resulting in a margin of 6.8% (Q1 2019: -3.0%).
Regional developments
In North America, revenue increased by 10% to EUR 3,186 million (+7% at constant currency, +3% organic). Operating income grew by 24% to EUR 463 million (+21% at constant currency), resulting in a margin of 14.5 % (Q1 2019: 12.9%). Despite the negative impact from the COVID-19 pandemic, the operating income margin increased mainly due to lower costs for pharmaceuticals and gains from divestitures.
EMEA revenue increased by 4% to EUR 679 million (+4% at constant currency, +3% organic). Operating income decreased by 27% to EUR 101 million (-27% at constant currency), resulting in a margin of 14.9% (Q1 2019: 21.1%). The decrease in operating income margin was mainly due to the prior year reduction of a contingent consideration liability related to Xenios.
In Asia-Pacific, revenue grew by 4% to EUR 443 million (+3% at constant currency, +2% organic). Operating income decreased by 19% to EUR 77 million (-20% at constant currency), resulting in a margin of 17.3 % (Q1 2019: 22.1). The decrease in operating income margin was mainly due to impacts from unfavorable foreign currency transaction effects, lower product sales as well as expansion into in-center dialysis services.
Latin America revenue increased by 4% to EUR 168 million (+24% at constant currency, +17% organic). Operating income decreased by 40% to EUR 7 million (-40% at constant currency), resulting in a margin of 4.1% (Q1 2019: 7.1%). The decrease in operating income margin was mainly due to unfavorable foreign currency impacts.
1 Net income attributable to shareholders of Fresenius Medical Care AG & Co. KGaA
2 For a reconciliation of adjusted figures, please refer to the table at the end of the press release
3 Special items are effects that are unusual in nature and have not been foreseeable or not
foreseeable in size or impact at the time of giving guidance.
Conference call
Fresenius Medical Care will host a conference call to discuss the results of the first quarter 2020 on May 6, 2020 at 3:30 p.m. CEDT (UTC +2) / 09:30 a.m. EDT (UTC -4). Details will be available on the company’s website www.freseniusmedicalcare.com in the “Investors” section. A replay will be available shortly after the call.
Please refer to our statement of earnings included at the end of this news and to the attachments as separate PDF-files for a complete overview of the results for the first quarter 2020. Our 6-K disclosure provides more details.
This release contains forward-looking statements that are subject to various risks and uncertainties. Actual results could differ materially from those described in these forward-looking statements due to various factors, including, but not limited to, changes in business, economic and competitive conditions, legal changes, regulatory approvals, results of clinical studies, foreign exchange rate fluctuations, uncertainties in litigation or investigative proceedings, and the availability of financing. These and other risks and uncertainties are detailed in Fresenius Medical Care AG & Co. KGaA's reports filed with the U.S. Securities and Exchange Commission. Fresenius Medical Care AG & Co. KGaA does not undertake any responsibility to update the forward-looking statements in this release.
Fresenius Medical Care North America is making 150 additional dialysis machines available to U.S. hospitals for the emergency treatment of COVID-19 patients, under the company’s newly established National Intensive Renal Care Reserve. The reserve creates a pool of dialysis machines that can be sent to hospitals on short notice, and nearly doubles the volume of consumables available to perform treatments. The increased demand is a result of many COVID-19 patients requiring renal replacement therapy due to acute kidney injury, and not all hospitals having enough dialysis machines to treat them.
Fresenius Medical Care, the world’s leading provider of dialysis products and services, has released an advisory calling for clinical care principles on access to be upheld for patients with advanced chronic kidney disease if a surge in COVID-19 infections overwhelms the available supply of facilities, caregivers and ventilators.
The Global Medical Advisory on Apportioning Care outlines the company’s position on how care should be allotted during and after the pandemic and addresses the differing care standards being articulated by various healthcare systems, groups and governments. It stresses the company’s commitment to maintaining uninterrupted healthcare delivery for patients around the world who require renal replacement therapy during the pandemic.
The advisory recognizes that capacity issues may become overwhelming to healthcare systems during the pandemic. However, it calls on “all authorities to recognize the need to make critical equipment, supplies, facilities and care delivery available to patients with advanced kidney diseases that need lifesaving therapy at all times, including during this COVID-19 disease pandemic.”
Dr. Frank Maddux, Global Chief Medical Officer of Fresenius Medical Care and member of the company’s Management Board, said the company stands firmly behind the clinical principle that despite the strain on healthcare resources caused by the pandemic, patients with End Stage Kidney Disease requiring dialysis should be able to continue with their prescribed dialysis treatment frequency. Furthermore, dialysis access surgery or interventions needed by some patients are “not elective procedures and should be delivered in a timely manner” to prevent potential complications, he added.
The advisory asserts that patients needing renal replacement therapy during the pandemic should still be offered the appropriate treatment that has been agreed in consultation with their physicians. “These discussions may include mechanical ventilation, dialysis, continuous renal replacement therapies and any other extracorporeal support” advised by a patient’s care team, it states.
“People with advanced kidney disease come in varied degrees of health and functional status, but they all want to be productive and active members of their families and communities, and we are doing everything in our power to support their desire for individual choices on the level of care they receive,” said Dr. Maddux. “We want to avoid population-based decisions on care that may not be in the best interests of the individual person.”
This release contains forward-looking statements that are subject to various risks and uncertainties. Actual results could differ materially from those described in these forward-looking statements due to certain factors, including changes in business, economic and competitive conditions, regulatory reforms, foreign exchange rate fluctuations, uncertainties in litigation or investigative proceedings, and the availability of financing. These and other risks and uncertainties are detailed in Fresenius Medical Care AG & Co. KGaA's reports filed with the U.S. Securities and Exchange Commission. Fresenius Medical Care AG & Co. KGaA does not undertake any responsibility to update the forward-looking statements in this release.
Fresenius Medical Care North America has announced a collaboration with DaVita Inc. and other dialysis providers in response to COVID-19, which aims to support the broader kidney care community in the United States by offering isolation capacity for dialysis patients who are or may be COVID-19 positive.
Dialysis patients represent one of the most at-risk populations, particularly in the current difficult and rapidly evolving situation. To stay alive, they must receive treatment multiple days a week for three to four hours at a time. This presents a unique challenge for patients and their care teams when social distancing is required to reduce the risk of community spread and infection.
In this unprecedented time, Fresenius Medical Care North America is cooperating with DaVita Inc., U.S. Renal Care, American Renal Associates, Satellite Healthcare and other dialysis organizations. Together they are creating a nationwide contingency plan with a goal of helping to maintain continuity of care for dialysis patients by creating isolation cohort capacity that can be accessed by other dialysis providers.
A critical aim of this collaboration is to keep dialysis patients out of the hospital whenever possible, freeing up limited hospital resources. The companies are focused on ensuring there are enough nurses, social workers, dietitians, care technicians and available space to treat all dialysis patients, including those who are or may be infected with COVID-19, in a way that does not unnecessarily expose the hundreds of thousands of other patients who entrust them with their care.
Dialysis organizations have designated capacity in selected clinics across their national networks to create isolation units and shifts, which will treat patients who are or may be COVID-19 positive separately. This collaboration will help safeguard caregivers, conserve personal protective equipment and other important supplies, and create an environment that provides excess capacity for providers that may be overwhelmed by larger COVID-19 clusters.
This release contains forward-looking statements that are subject to various risks and uncertainties. Actual results could differ materially from those described in these forward-looking statements due to various factors, including, but not limited to, changes in business, economic and competitive conditions, legal changes, regulatory approvals, results of clinical studies, foreign exchange rate fluctuations, uncertainties in litigation or investigative proceedings, and the availability of financing. These and other risks and uncertainties are detailed in Fresenius Medical Care AG & Co. KGaA's reports filed with the U.S. Securities and Exchange Commission. Fresenius Medical Care AG & Co. KGaA does not undertake any responsibility to update the forward-looking statements in this release.
Fresenius Medical Care, the world’s leading provider of dialysis products and services, postpones its 2020 Annual General Meeting, which was scheduled for May 19, to a later date this year due to the coronavirus pandemic. The resolutions regarding the allocation of the distributable profit and the payout of the dividend will be postponed accordingly.
As soon as conditions allow again for a reliable planning and safe implementation of the Annual General Meeting, Fresenius Medical Care will announce the new date.
Rice Powell, Chief Executive Officer of Fresenius Medical Care, said: “The decision to postpone our Annual General Meeting was not an easy one for us to take. But the protection and health of our shareholders and employees is very important to us. In light of this exceptional situation, a postponement is the only sensible option.”
This release contains forward-looking statements that are subject to various risks and uncertainties. Actual results could differ materially from those described in these forward-looking statements due to certain factors, including changes in business, economic and competitive conditions, regulatory reforms, foreign exchange rate fluctuations, uncertainties in litigation or investigative proceedings, and the availability of financing. These and other risks and uncertainties are detailed in Fresenius Medical Care AG & Co. KGaA's reports filed with the U.S. Securities and Exchange Commission. Fresenius Medical Care AG & Co. KGaA does not undertake any responsibility to update the forward-looking statements in this release.

Fresenius Medical Care, the world’s leading provider of dialysis products and services, today announced that the U.S. Food and Drug Administration (FDA) has cleared Novalung®, a heart and lung support system for the treatment of acute respiratory or cardiopulmonary failure. Novalung is the first extracorporeal membrane oxygenation (ECMO) system to be cleared for more than six hours of use as extracorporeal life support. The company expects Novalung to be available within the U.S. mid-year 2020.
Patients in acute respiratory or cardiopulmonary failure often struggle to get oxygen into their bloodstream or expel carbon dioxide out of their bodies, resulting in dangerously low levels of oxygen. This acute low oxygen state can result from a wide range of conditions.
The Novalung ECMO system pumps and oxygenates a patient’s blood, reducing the stress on damaged heart and lungs. Novalung offers a portable therapy solution designed to improve clinical outcomes and accommodate various clinical care settings such as intensive care units, operating rooms, cardiac catheterization labs, and emergency departments.
The use of Novalung as an ECMO device for critical care has several benefits, including minimizing the need for invasive ventilation, the ability to provide support after multi-organ injuries, and better survival outcomes for patients in cardiac arrest.
This release contains forward-looking statements that are subject to various risks and uncertainties. Actual results could differ materially from those described in these forward-looking statements due to certain factors, including changes in business, economic and competitive conditions, regulatory reforms, foreign exchange rate fluctuations, uncertainties in litigation or investigative proceedings, and the availability of financing. These and other risks and uncertainties are detailed in Fresenius Medical Care AG & Co. KGaA's reports filed with the U.S. Securities and Exchange Commission. Fresenius Medical Care AG & Co. KGaA does not undertake any responsibility to update the forward-looking statements in this release.
- Solid Q4 performance
- Full year guidance achieved
- Investments in home dialysis and growth markets as planned
- Cost optimization initiatives as planned
- 23rd dividend increase proposed
- Outlook 2020 confirmed
Rice Powell, Chief Executive Officer of Fresenius Medical Care, said: “2019 was a successful year for Fresenius Medical Care. We achieved our revenue and net income targets and are therefore proposing our 23rd consecutive dividend increase. Last year we also invested more strongly in our future growth, particularly in the area of home dialysis and in developing economies. In addition, our measures to increase efficiency and optimize our cost base are progressing according to plan. As a consequence, we expect growth to accelerate, and confirm the 2020 outlook that we issued early last year.”
2020 guidance confirmed: mid to high single digit growth rates
Fresenius Medical Care expects both revenue and net income to grow at a mid to high single digit rate in 2020. These targets are in constant currency and exclude special items3 and are based on the adjusted results 2019 including the effects of the operations of the NxStage acquisition and the IFRS 16 implementation.
Investment year 2019
The continued expansion of home dialysis in the U.S. is a key growth area for Fresenius Medical Care. As announced at the beginning of 2019, our investments focused on home training facilities, educational staff and materials along with scaling the distribution infrastructure to support both products and services. The closing of the acquisition of NxStage Medical, Inc. in February 2019 marked a milestone in our home dialysis strategy. In 2019, Fresenius Medical Care reported record growth with more than 25,000 patients being treated at home in North America.
The second major focus of investment were developing economies. In China, the world’s fastest-growing dialysis market, Fresenius Medical Care invested in expanding production capacities, research and development activities as well as in strengthening its services business, that has almost doubled with now 11 clinics. In September 2019, we launched the 4008A dialysis machine in China, laying an important foundation for the further development of the market.
In addition, Fresenius Medical Care invested EUR 91 million (EUR 83 million in North America) to sustainably improve the cost base of our clinical infrastructure. This 2019 cost optimization program is expected to be accretive to net income from the current financial year onwards. In 2019, we achieved further sustained cost improvements as part of our Global Efficiency Program (GEP II), in line with the originally anticipated contribution.
Creating shareholder value
Based on the solid results for 2019, the General Partner and the Supervisory Board will propose a new record dividend of EUR 1.20 per share, corresponding to a total payout of EUR 358 million, to the Annual General Meeting in May 2020. This proposal would result in the 23rd consecutive dividend increase.
In order to create additional shareholder value, Fresenius Medical Care launched a share buy-back program in 2019. Between March and December 2019, 8.9 million own shares were bought back at a total purchase price of EUR 600 million. The Company intends to use its residual authorization of EUR 400 million over the course of 2020.
Patients, Clinics and Employees
As of December 31, 2019, Fresenius Medical Care treated 345,096 patients in 3,994 dialysis clinics worldwide. At the end of 2019, Fresenius Medical Care had 120,659 employees (full-time equivalents) worldwide, compared to 112,658 employees as of December 31, 2018.
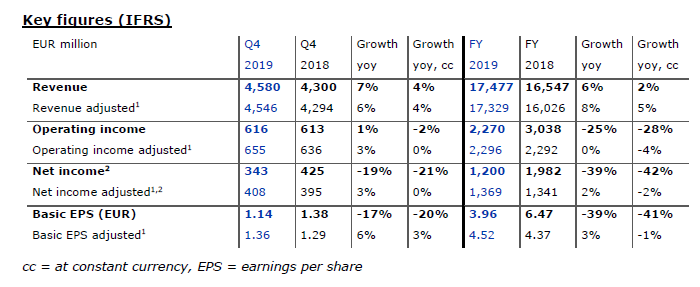
Strong organic revenue growth continued
Revenue for the fourth quarter of 2019 increased by 7% (+4% at constant currency) to EUR 4,580 million. Organic growth remained strong at 5%4. Adjusted revenue increased by 6% (+4% at constant currency) to EUR 4,546 million. For a detailed reconciliation, please refer to the table at the end of the press release.
Health Care Services revenue rose by 6% to EUR 3,607 million (+3% at constant currency), while Health Care Products revenue grew by 10% to EUR 973 million (+8% at constant currency). This includes the negative effect from a revenue recognition adjustment of EUR 86 million (FY 2019: EUR 170 million) for accounts receivable in legal dispute in North America.
Revenue for the full year 2019 rose by 6% to EUR 17,477 million (+2% at constant currency). Organic growth amounted to 5%. Adjusted revenue increased by 8% (+5% at constant currency) to EUR 17,329 million. Health Care Services revenue grew by 5% (+1% at constant currency) to EUR 13,872 million. Growth in same market treatments, contributions from acquisitions and increases in organic revenue per treatment were partly offset by decreases attributable to prior year revenue from divested activities of Sound Physicians and closed or sold clinics. Health Care Products revenue for the full year 2019 rose by 10% to EUR 3,605 million (+8% at constant currency). This increase was mainly driven by higher sales of home dialysis products as a result of the NxStage acquisition and by higher sales of dialyzers. This was partially offset by lower sales of machines as a result of changes in the accounting treatment for sale-leaseback transactions due to the IFRS 16 implementation.
In the fourth quarter operating income increased by 1% to EUR 616 million (-2% at constant currency), resulting in a margin of 13.5% (Q4 2018: 14.3%). Adjusted operating income grew by 3% to EUR 655 million (stable at constant currency), resulting in a margin of 14.4% (Q4 2018: 14.8%). The included negative effect from a revenue recognition adjustment for accounts receivable in legal dispute in North America is EUR 86 million (FY 2019: EUR 170 million). For a detailed reconciliation, please refer to the table at the end of the press release.
Operating income for the full year decreased by 25% to EUR 2,270 million (-28% at constant currency), resulting in a margin of 13.0% (FY 2018: 18.4%). The 2018 basis includes the gain from the divestiture of Care Coordination activities including Sound Physicians. On an adjusted basis, operating income remained stable at EUR 2,296 million (-4% at constant currency), resulting in a margin of 13.2% (FY 2018: 14.3%).
Net income2 for the fourth quarter decreased by 19% to EUR 343 million (-21% at constant currency). Adjusted net income2 increased by 3% to EUR 408 million (+0% at constant currency). For a detailed reconciliation, please refer to the table at the end of the press release. Basic earnings per share (EPS) decreased by 17% to EUR 1.14 (-20% at constant currency). On an adjusted basis, EPS increased by 6% to EUR 1.36 (+3% at constant currency).
For the full year, net income decreased by 39% to EUR 1,200 million (-42% at constant currency). EPS decreased by 39% to EUR 3.96 (-41% at constant currency). Here, too, the 2018 basis includes the gain from the divestiture of Care Coordination activities including Sound Physicians. On an adjusted basis, net income grew by 2% to EUR 1,369 million (-2% at constant currency). This resulted in a 3% increase in adjusted EPS to EUR 4.52 (-1% at constant currency).
Strong Cash-flow development
In the fourth quarter, Fresenius Medical Care generated EUR 771 million of operating cash flow (Q4 2018: EUR 698 million) resulting in a margin of 16.8% (Q4 2018: 16.2%). The increase was largely driven by the IFRS 16 implementation. Free cash flow (net cash used in operating activities, after capital expenditures, before acquisitions and investments) amounted to EUR 434 million (Q4 2018: EUR 397 million) resulting in a margin of 9.5% (Q4 2018: 9.2%).
In the full year, we generated operating cash flow of EUR 2,567 million resulting in a margin of 14.7% (FY 2018: EUR 2,062 million, 12.5%). The increase was mainly due to the IFRS 16 implementation. Free cash flow for the full year 2019 amounted to EUR 1,454 million resulting in a margin of 8.3% (FY 2018: EUR 1,059 million, 6.4%).
Regional developments
In North America, revenue in the fourth quarter of 2019 increased by 6% to EUR 3,174 million (+3% at constant currency, +5% organic growth). For the full year 2019, North America revenue rose by 5% to EUR 12,195 million (stable at constant currency, +4% organic).
Operating income for the fourth quarter grew by 5% to EUR 515 million (+2% at constant currency). For the full year, operating income decreased by 33% to EUR 1,794 million (-36% at constant currency). The 2018 basis includes the gain from the divestiture of Care Coordination activities including Sound Physicians.
In EMEA, revenue in the fourth quarter increased by 4% to EUR 709 million (+4% at constant currency, +3% organic). For the full year, EMEA revenue rose by 4% to EUR 2,693 million (+4% at constant currency, +4% organic).
Operating income for the fourth quarter rose by 17% to EUR 114 million (+17% at constant currency). For the full year, operating income grew by 12% to EUR 448 million (+13% at constant currency) resulting in a margin of 16.6% (FY 2018: 15.4%). This improvement was mainly driven by a reduction of a contingent consideration liability related to Xenios and a positive impact from the IFRS 16 implementation.
In Asia-Pacific, revenue in the fourth quarter 2019 grew by 10% to EUR 499 million (+7% at constant currency, +6% organic). For the full year, revenue increased by 10% to EUR 1,859 million (+7% at constant currency, +7% organic).
Operating income for the fourth quarter decreased by 13% to EUR 75 million (-14% at constant currency). The decline in margin in the fourth quarter was mainly due to investments in business growth and expenses for the cost optimization program. For the full year, operating income grew by 8% to EUR 329 million (+6% at constant currency).
Regarding the coronavirus (nCoV) outbreak the first priority is to ensure continuation of treatments for our patients and the safety of our employees. It is too early to quantify the potential impact to our Asia-Pacific operations.
In Latin America, revenue for the fourth quarter increased by 6% to EUR 193 million (+24% at constant currency, +19% organic). For the full year, Latin America revenue increased by 3% (+21% at constant currency, +17% organic) to EUR 709 million.
Operating income for the fourth quarter increased by 189% to EUR 15 million (+201% at constant currency). For the full year, operating income increased by 47% to EUR 43 million (+35% at constant currency). The improved margin was mainly due to favorable foreign currency transaction effects.
1 For a detailed reconciliation, please refer to the table at the end of the press release.
2 Net income attributable to shareholders of Fresenius Medical Care AG & Co. KGaA
3 Special items are effects that are unusual in nature and have not been foreseeable or not foreseeable in size or impact at the time of giving guidance.
4 Definition of organic growth excludes effects such as out of period adjustments or effects from IFRS 16 implementation.
Press conference
Fresenius Medical Care will hold a press conference at its headquarters in Bad Homburg, Germany to discuss the results of the fourth quarter and full year tomorrow on Thursday, February 20, 2020, at 10:00 a.m. CET / 4:00a.m. EST. The press conference will be webcasted on the company’s website www.freseniusmedicalcare.com in the “Media” section. A replay will be available shortly after the conference.
Conference call
We will also host a conference call to discuss the results of the fourth quarter tomorrow on Thursday, February 20, 2020, at 3:30 p.m. CET / 09:30 a.m. EST. Details will be available on the company’s website www.freseniusmedicalcare.com in the “Investors” section. A replay will be available shortly after the call.
Please refer to our statement of earnings included at the end of this news and to the attachments as separate PDF-files for a complete overview of the results for the fourth quarter and full year 2019. Our 20-F disclosure provides more details.
Disclaimer: This release contains forward-looking statements that are subject to various risks and uncertainties. Actual results could differ materially from those described in these forward-looking statements due to various factors, including, but not limited to, changes in business, economic and competitive conditions, legal changes, regulatory approvals, results of clinical studies, foreign exchange rate fluctuations, uncertainties in litigation or investigative proceedings, and the availability of financing. These and other risks and uncertainties are detailed in Fresenius Medical Care AG & Co. KGaA's reports filed with the U.S. Securities and Exchange Commission. Fresenius Medical Care AG & Co. KGaA does not undertake any responsibility to update the forward-looking statements in this release.
The information and documents contained on the following pages of this website are for information purposes only. These materials do neither constitute an offer nor an invitation to subscribe to or to purchase securities, nor any investment advice or service, and are not meant to serve as a basis for any kind of obligation, contractual or otherwise. Securities may not be offered or sold in the United States of America (“US”) absent registration under the US Securities Act of 1933, as amended, or an exemption from registration. The securities described on the following pages are not offered for sale in the US or to "US persons" (as defined in Regulation S under the US Securities Act of 1933, as amended).
THE FOLLOWING INFORMATION AND DOCUMENTS ARE NOT DIRECTED AT AND ARE NOT INTENDED FOR USE BY (I) PERSONS WHO ARE RESIDENTS OF OR LOCATED IN THE US, CANADA, JAPAN OR AUSTRALIA OR WHO ARE US PERSONS (AS DEFINED IN REGULATION S UNDER THE US SECURITIES ACT OF 1933, AS AMENDED), OR (II) PERSONS IN ANY OTHER JURISDICTION WHERE THE COMMUNICATION OR RECEIPT OF SUCH INFORMATION IS RESTRICTED IN SUCH A WAY THAT PROVIDES THAT SUCH PERSONS SHALL NOT RECEIVE IT. SUCH PERSONS, OR PERSONS ACTING FOR THE BENEFIT OF ANY SUCH PERSONS, ARE NOT PERMITTED TO VISIT THE FOLLOWING PAGES OF THE WEBSITE.
To visit the following parts of this website you must confirm that
(i) you are not a resident of the United States of America, Canada, Japan or Australia or a "US person" (as defined in Regulation S under the US Securities Act of 1933, as amended),
(ii) you are not a person to whom the communication of the information contained on the website is restricted,
(iii) you will not distribute any of the information and documents contained thereon to any such person, and
(iv) you are not acting for the benefit of any such person.
By clicking on the "Accept" button below, you will be deemed to have made this confirmation.
NOT FOR RELEASE, PUBLICATION OR DISTRIBUTION, DIRECTLY OR INDIRECTLY, IN OR INTO THE UNITED STATES OF AMERICA, AUSTRALIA, CANADA OR JAPAN.
Fresenius Medical Care, the world’s largest provider of dialysis products and services, today successfully placed bonds in three tranches with an aggregate volume of €1.75 billion:
- €650 million bonds with a 4-year maturity and a coupon of 0.250% were issued at a price of 99.901% resulting in a yield of 0.275%,
- €600 million bonds with a 7-year maturity and a coupon of 0.625% were issued at a price of 99.238% resulting in a yield of 0.737%,
- €500 million bonds with a 10-year maturity and a coupon of 1.250% were issued at a price of 99.832% resulting in a yield of 1.268%.
The proceeds will be used for general corporate purposes, including refinancing of existing financial liabilities.
The bonds were drawn under the European Medium Term Note (EMTN) Program by Fresenius Medical Care. The company has applied to the Luxembourg Stock Exchange to admit the bonds to trading on its regulated market.
Disclaimer
This announcement does not contain or constitute an offer of, or the solicitation of an offer to buy or subscribe for, securities of Fresenius Medical Care to any person in Australia, Canada, Japan, or the United States of America (the “United States”) or in any jurisdiction to whom or in which such offer or solicitation is unlawful. The securities referred to herein have not been and will not be registered under U.S. Securities Act of 1933, as amended (the “Securities Act”) and may not be offered or sold in the United States or to, or for the account or benefit of, U.S. persons, absent such registration, except pursuant to an exemption from, or in a transaction not subject to, the registration requirements of the Securities Act. Subject to certain exceptions, the securities referred to herein may not be offered or sold in Australia, Canada or Japan or to, or for the account or benefit of, any national, resident or citizen of Australia, Canada or Japan. The offer and sale of the securities referred to herein has not been and will not be registered under the Securities Act or under the applicable securities laws of Australia, Canada or Japan. There will be no public offer of the securities in the United States.
This announcement neither constitutes an offer to sell nor a solicitation to buy any securities of Fresenius Medical Care. The securities referred to in this announcement have already been sold.
No action has been taken that would permit an offering of the securities or possession or distribution of this announcement in any jurisdiction where action for that purpose is required. Persons into whose possession this announcement comes are required to inform themselves about and to observe any such restrictions. This announcement has been prepared on the basis that any offer of Notes in any Member State of the European Economic Area (EEA) which has implemented the Prospectus Directive (2003/71/EC), as amended (each, a Relevant Member State) will be made pursuant the prospectus prepared by Fresenius Medical Care AG & Co. KGaA or pursuant to an exemption under the Prospectus Directive, as implemented in that Relevant Member State, from the requirement to publish a prospectus for offers of securities. Fresenius Medical Care AG & Co. KGaA has not authorized, nor does it authorize, the making of any offer of securities in circumstances in which an obligation arises for Fresenius Medical Care AG & Co. KGaA or any other person to publish or supplement a prospectus for such offer.
This announcement is directed at and/or for distribution in the United Kingdom only to (i) persons who have professional experience in matters relating to investments falling within article 19(5) of the Financial Services and Markets Act 2000 (Financial Promotion) Order 2005 (the “Order”) or (ii) high net worth entities falling within article 49(2)(a) to (d) of the Order (all such persons are referred to herein as “relevant persons”). This announcement is directed only at relevant persons. Any person who is not a relevant person should not act or rely on this announcement or any of its contents. Any investment or investment activity to which this announcement relates is available only to relevant persons and will be engaged in only with relevant persons.
This announcement contains forward-looking statements that are subject to various risks and uncertainties. Future results could differ materially from those described in these forward-looking statements due to certain factors, e.g. changes in business, economic and competitive conditions, regulatory reforms, results of clinical trials, foreign exchange rate fluctuations, uncertainties in litigation or investigative proceedings, and the availability of financing. Fresenius Medical Care does not undertake any responsibility to update the forward-looking statements in this announcement.
The information contained in this announcement is for background purposes only and does not purport to be full or complete. No reliance may be placed for any purpose on the information contained in this announcement or its accuracy or completeness. The information in this announcement is subject to change.
Fresenius Medical Care North America (FMCNA) has launched its new connected health platform called TheHub. The platform is comprised of three integrated applications - PatientHub, CareTeamHub and ProviderHub - that enable patients, care teams, and providers to better collaborate and monitor patient treatments. Research by FMCNA indicates that patients who actively use these connected health solutions have a 20 percent lower risk of hospitalization, higher transplantation rates, and stay on the modality longer.
Fresenius Medical Care North America continues to improve clinical quality as measured by the U.S. government’s Five-Star Rating System. More than 94 percent of the company’s dialysis centers were rated three stars and above for clinical quality, with a total of 762 centers achieving the highest five star rating, up from 658 last year. The Centers for Medicare and Medicaid Services (CMS) assigns one to five stars to each dialysis center annually based on a series of measurements around clinical performance and patient outcomes.



