- Stronger than projected headwind from COVID-19 effects with significantly increased patient excess mortality due to global spread of Delta variant
- Organic growth continued with 1%
- Financial targets for FY 2021 confirmed, expectation to reach lower end of the guidance ranges for revenue and net income
- FME25 transformation update to achieve EUR 500 million savings by 2025
Rice Powell, Chief Executive Officer of Fresenius Medical Care, said: “The COVID-19 pandemic is an unprecedented situation for all of us and still costs many lives day by day. The existence of the Delta variant has caused excess mortality among our patients to rise again in the third quarter. This means that we must absorb a sizably larger COVID-19 effect on our business than we projected at the beginning of the year and, at the same time, manage through an increasingly inflationary cost environment. Against this backdrop, we now expect to reach the lower end of our guidance ranges for both revenue and net income.”
Spread of Delta variant caused resurgence of excess mortality
As a result of the global spread of the Delta variant, COVID-19-related incremental excess mortality3 among Fresenius Medical Care’s patients has increased again to around 2,700 in the third quarter of 2021 (Q1 2021: ~3,200; Q2 2021: ~1,900). Thus, excess mortality amounted to approximately 11,500 patients in the past 12 months (as of September 30, 2021) and approximately 18,200 since the start of the pandemic.
The increase in excess mortality in the third quarter has led to a more negative impact on Fresenius Medical Care’s growth compared to the second quarter. The overall adverse effect of accumulated COVID-19-related excess mortality on organic growth in the Health Care Services business amounted to around 390 basis points in the third quarter and 330 basis points in the first nine months of 2021.
At the end of the third quarter, approximately 78% of Fresenius Medical Care’s patients in the U.S. had received at least one dose of COVID-19 vaccine. Also on a global basis, approximately 78% of Fresenius Medical Care’s patients have received at least one vaccination.
1 Costs related to the FME25 program
2 Net income attributable to shareholders of Fresenius Medical Care AG & Co. KGaA
3 Historical excess mortality updated for late entries
Outlook
When giving guidance in February 2021, Fresenius Medical Care assumed an accumulation of COVID-19-related excess mortality in the first half and a return to normal mortality patterns in the second half of the year. The continued and increasing presence of COVID-19 has led to a significant increase in excess mortality in the third quarter which was not included in the Company’s outlook for 2021. Due to decreasing infection rates since the end of the third quarter, the Company is now projecting excess mortality to decline in the fourth quarter.
Based on Fresenius Medical Care’s current projections, the Company confirms its outlook for revenue to grow at a low- to mid-single digit percentage rate and net income to decline at a high-teens to mid-twenties percentage rate against the 2020 base and is now expecting to be at the lower end of these guidance ranges.4
4 These targets are based on the 2020 results excluding the impairment of goodwill and trade names in the Latin America Segment of EUR 195 million. They are inclusive of anticipated COVID-19 effects, in constant currency and exclude special items. Special items include costs related to FME25 and other effects that are unusual in nature and have not been foreseeable or not foreseeable in size or impact at the time of giving guidance.
Revenue and earnings development affected by COVID-19 pandemic
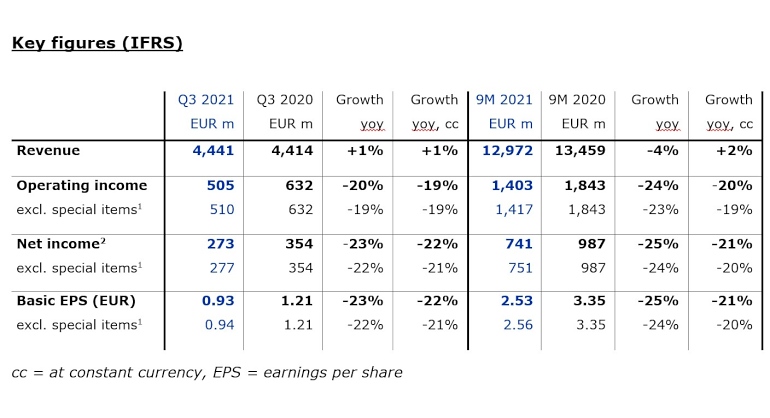
Revenue in the third quarter increased by 1% to EUR 4,441 million (+1% at constant currency, +1% organic).
Health Care Services revenue in the third quarter increased by 1% to EUR 3,530 million (+2% at constant currency, +1% organic). Organic growth was achieved despite the adverse effects of the COVID-19 pandemic and a lower reimbursement for calcimimetics.
Health Care Products revenue was stable and amounted to EUR 911 million (-1% at constant currency, 0% organic). Lower sales of in-center disposables and peritoneal dialysis products were offset by positive exchange rate effects and higher sales of machines for chronic treatment.
In the first nine months, revenue declined by 4% to EUR 12,972 million (+2% at constant currency, +1% organic). Health Care Services revenue decreased by 4% to
EUR 10,255 million (+2% at constant currency, +1% organic); Health Care Products revenue declined by 1% to EUR 2,717 million (+2% at constant currency, +2% organic).
Operating income decreased by 20% to EUR 505 million in the third quarter (-19% at constant currency), resulting in a margin of 11.4% (Q3 2020: 14.3%). Operating income excluding special items declined by 19% to EUR 510 million (-19% at constant currency), resulting in a margin of 11.5% (Q3 2020: 14.3%). The decline was mainly due to adverse COVID-19-related effects, inflationary materials cost increases and higher labor costs. These effects were slightly mitigated by an improved U.S. payor mix, in particular due to an increased number of patients with Medicare Advantage coverage.
Operating income for the first nine months decreased by 24% to EUR 1,403 million (-20% at constant currency), resulting in a margin of 10.8% (9M 2020: 13.7%). Operating income excluding special items declined by 23% to EUR 1,417 million (-19% at constant currency), resulting in a margin of 10.9% (9M 2020: 13.7%).
Net income2 decreased by 23% to EUR 273 million in the third quarter (-22% at constant currency) mainly due to the explained effects on operating income and a higher tax rate. Net income excluding special items declined by 22% to EUR 277 million (-21% at constant currency).
In the first nine months, net income decreased by 25% to EUR 741 million (-21% at constant currency). Net income excluding special items declined by 24% to EUR 751 million (-20% at constant currency).
Basic earnings per share (EPS) decreased by 23% to EUR 0.93 (-22% at constant currency) in the third quarter. EPS excluding special items declined by 22% to EUR 0.94 (-21% at constant currency).
In the first nine months, EPS decreased by 25% to EUR 2.53 (-21% at constant currency). EPS excluding special items declined by 24% to EUR 2.56 (-20% at constant currency).
Cash flow development
In the third quarter, Fresenius Medical Care generated EUR 692 million of operating cash flow (Q3 2020: EUR 746 million), resulting in a margin of 15.6% (Q3 2020: 16.9%). The decrease was mainly due to continued recoupment of the U.S. federal government’s payments in the second quarter of 2020 under the CARES Act. In the first nine months, operating cash flow amounted to EUR 1,820 million (9M 2020: EUR 3,649 million), resulting in a margin of 14.0% (9M 2020: 27.1%).
Fresenius Medical Care generated EUR 511 million of free cash flow5 (Q3 2020: EUR 507 million) in the third quarter, resulting in a margin of 11.5% (Q3 2020: 11.5%). In the first nine months, Free cash flow amounted to EUR 1,259 million (9M 2020: EUR 2,913 million), resulting in a margin of 9.7% (9M 2020: 21.6%).
5 Net cash provided by / used in operating activities, after capital expenditures, before acquisitions, investments, and dividends
Regional developments
In North America, revenue was stable and amounted to EUR 3,080 million in the third quarter (+1% at constant currency, +1% organic). The low growth was mainly due to the adverse COVID-19 impact on both the Health Care Services and Health Care Products businesses along with associated downstream and negative exchange rate effects. These effects were partially offset by contributions from acquisitions. In the first nine months, revenue decreased by 6% to EUR 8,931 million (0% at constant currency, 0% organic).
Operating income in North America decreased by 13% to EUR 446 million in the third quarter (-13% at constant currency), resulting in a margin of 14.5% (Q3 2020: 16.8%). The decline was mainly due to the adverse impact of COVID-19, including a high prior-year base mainly driven by government relief funding, as well as inflationary cost increases. This was partially offset by an improved U.S. payor mix, in particular due to an increased number of patients with Medicare Advantage coverage. In the first nine months, operating income declined by 22% to EUR 1,242 million (-17% at constant currency), resulting in a margin of 13.9% (9M 2020: 16.7%).
Revenue in the EMEA region decreased by 2% to EUR 671 million in the third quarter (-2% at constant currency, -2% organic). The decrease was mainly due to adverse COVID-19-related impacts in Health Care Services as well as lower sales of in-center disposables and peritoneal dialysis products, partially offset by higher sales of products for acute care treatment and machines for chronic treatment. In the first nine months, revenue declined by 1% to EUR 2,033 million (+1% at constant currency, 0% organic).
Operating income in EMEA decreased by 21% to EUR 79 million in the third quarter (-21% at constant currency), resulting in a margin of 11.7% (Q3 2020: 14.6%). The decline was mainly due to inflationary cost increases, adverse COVID-19-related effects, an unfavorable mix in the Products business and timing of export sales. This was partially offset by favorable transactional exchange rate effects and an increase in reimbursement rates in certain countries. In the first nine months, operating income decreased by 17% to EUR 232 million (-16% at constant currency), resulting in a margin of 11.4% (9M 2020: 13.6%).
In Asia-Pacific, revenue increased by 4% to EUR 501 million in the third quarter (+3% at constant currency, +2% organic). This was mainly driven by organic growth in Health Care Services, including a recovery in elective procedures, as well as positive exchange rate effects, contributions from acquisitions and higher sales of machines for chronic treatment, partially offset by lower sales of in-center disposables. In the first nine months, revenue grew by 6% to EUR 1,458 million (+8% at constant currency, +8% organic).
Operating income decreased by 11% to EUR 86 million in the third quarter (-10% at constant currency), resulting in a margin of 17.2% (Q3 2020: 20.0%). The decline was mainly due to adverse COVID-19-related effects and inflationary cost increases, partially offset by business growth including the above-mentioned effect from the recovery of elective procedures. In the first nine months, operating income grew by 8% to
EUR 256 million (+10% at constant currency), resulting in a margin of 17.5% (9M 2020: 17.2%).
Despite a significant headwind from exchange rates and the adverse impact of COVID-19, Latin America revenue increased by 5% to EUR 178 million in the third quarter (+13% at constant currency, +12% organic). This was mainly driven by organic growth in the Services business, contributions from acquisitions and higher sales of in-center disposables. In the first nine months, revenue was stable and amounted to EUR 508 million (+16% at constant currency, +14% organic).
Operating income in Latin America decreased by 61% to EUR 4 million in the third quarter (-62% at constant currency), resulting in a margin of 2.4% (Q3: 2020: 6.6%). The decline was due to inflationary cost increases and adverse COVID-19 effects. In the first nine months, operating income declined by 53% to EUR 14 million (-54% at constant currency), resulting in a margin of 2.7% (9M 2020: 5.7%).
Patients, clinics and employees
As of September 30, 2021, Fresenius Medical Care treated 344,872 patients in 4,151 dialysis clinics worldwide. At the end of the third quarter, the Company had 123,528 employees (full-time equivalents) worldwide, compared to 126,463 employees as of September 30, 2020.
FME25 Update
For the update on the FME25 transformation program, a separate press release is issued.
Conference call
Fresenius Medical Care will host a two-hour conference call to discuss the results of the third quarter and first nine months of 2021 on November 2, 2021 at 3:30 p.m. CET / 10:30 a.m. EDT. Please note that this will be an extended call, as the Company will additionally provide an update on the FME25 transformation program. Details will be available on the Fresenius Medical Care website www.freseniusmedicalcare.com in the “Investors” section. A replay will be available shortly after the call.
Please refer to our statement of earnings included in the PDF file for a complete overview of the results of the third quarter and first nine months of 2021. Our 6-K disclosure provides more details.
This release contains forward-looking statements that are subject to various risks and uncertainties. Actual results could differ materially from those described in these forward-looking statements due to various factors, including, but not limited to, changes in business, economic and competitive conditions, legal changes, regulatory approvals, impacts related to the COVID-19 pandemic, results of clinical studies, foreign exchange rate fluctuations, uncertainties in litigation or investigative proceedings, and the availability of financing. These and other risks and uncertainties are detailed in Fresenius Medical Care AG & Co. KGaA's reports filed with the U.S. Securities and Exchange Commission. Fresenius Medical Care AG & Co. KGaA does not undertake any responsibility to update the forward-looking statements in this release.

Fresenius Medical Care, the world’s leading provider of products and services for individuals with renal diseases, has conducted a study examining the effectiveness of the “Medical Patient Review continuous quality improvement” (MPR-CQI) program in 20 countries of the region Europe, Middle East and Africa (EMEA). The study has demonstrated, by means of mediation analysis, that improvements in medical key performance indicators (KPIs), occurring after the MPR-CQI program implementation, was associated with a significant reduction in mortality risk of about 30 percent in study participants.
For the development of the MPR-CQI program, the company used therapy data from more than 70,000 patients treated in Fresenius Medical Care’s EMEA NephroCare network to define, monitor and improve 10 KPIs – intermediate clinical endpoints – including hemoglobin, hydration status, hemodynamic status and infusion volume. The Medical Patient Review’s monthly review circles and evaluations of patients’ health indicators allow physicians to identify, prioritize and address patient’s medical needs.
“Delivering the highest quality care for people living with kidney disease is central to our mission, and data-driven insights are vital for ongoing clinical care improvement and innovation,” said Franklin W. Maddux, MD, Global Chief Medical Officer of Fresenius Medical Care. “The breadth and depth of our company’s clinical data, combined with our efforts in digital innovation and connected health, are generating a compelling evolution of clinical results.”
The quality performance of Fresenius Medical Care’s clinical governance strategy has been evaluated in an historical cohort study based on the company's own EuCliD database in the EMEA region. The company has published the study results in Nephrology, Dialysis & Transplantation, the official journal of the European Renal Association/European Dialysis and Transplant Association, to make them publicly available to the professional community and support further improvements in care worldwide: https://doi.org/10.1093/ndt/gfab160
The Medical Patient Review continuous quality improvement program is based on EuCliD data – Fresenius Medical Care’s digital patient care system- and enables the company to define evaluation processes involving medical and nursing experts. It also enables a detailed analysis of patients that allows a better understanding of medical needs, while providing intensive experiences from correlating improvements in medical and economic efficiency results – to deliver feedback focused on effective and efficient care. Through its digital patient care system, Fresenius Medical Care is planning to also enable third-party clients to benefit from the MPR.
As part of its 2025 growth strategy, Fresenius Medical Care is using digital technologies and artificial intelligence (AI) to develop new forms of kidney therapy. Physicians in more than 100 clinics in the company’s network can already use an AI-based algorithm that can help to improve renal anemia management while reducing drug-related costs. Fresenius Medical Care is planning to enrich the Medical Patient Review process by the MPR Advanced Benchmarking System which is based on an ensemble of 22 AI-based models that are designed to help detect best clinical practices, pinpoint high-performing centers beyond case-mix differences, discover emerging medical needs and set realistic margins of improvement for each intermediate clinical endpoint in all clinics.
The MPR Advanced Benchmarking System will be piloted starting this fall in NephroCare clinics in one EMEA country. Additional AI-based products for the management of non-dialysis dependent chronic kidney disease, vascular access care, home dialysis therapies and intradialytic complications are now being developed.
This release contains forward-looking statements that are subject to various risks and uncertainties. Actual results could differ materially from those described in these forward-looking statements due to various factors, including, but not limited to, changes in business, economic and competitive conditions, legal changes, regulatory approvals, impacts related to the COVID-19 pandemic results of clinical studies, foreign exchange rate fluctuations, uncertainties in litigation or investigative proceedings, and the availability of financing. These and other risks and uncertainties are detailed in Fresenius Medical Care AG & Co. KGaA's reports filed with the U.S. Securities and Exchange Commission. Fresenius Medical Care AG & Co. KGaA does not undertake any responsibility to update the forward-looking statements in this release.
Fresenius Medical Care, the world's leading provider of products and services for individuals with renal diseases, has donated 250,000 euros to UNICEF, the United Nations Children's Fund, to mark the company’s 25th anniversary. UNICEF will use the donation to support the COVAX vaccination initiative to fight the COVID-19 pandemic in about 140 countries. UNICEF delivers COVID-19 vaccines and other equipment around the world. With 250,000 euros UNICEF is able, for example, to protect almost 70,000 teachers or medical personnel from a COVID-19 infection. With every health worker who is protected from the virus the care of numerous children can be ensured. With every vaccinated teacher, more and more children can return to school.
Rice Powell, CEO of Fresenius Medical Care, presented a symbolic check at a virtual ceremony. “As a company in the healthcare branch, we bear a special responsibility to our patients, to our employees and as a member of our society as well,” he said. “In this anniversary year, we must do everything to defeat the global corona pandemic. We are doing this by various means. Our donation to UNICEF is targeted to protect the health of children in less developed and in developing countries. This lives up to our values and our mission for the past 25 years to maintain life and improve the quality of life.”
As the world’s largest single buyer of vaccines, UNICEF has unique experience, gained over many years, in purchasing and delivering goods to children in need. Working on behalf of nearly 100 countries, UNICEF procures more than 2 billion vaccine doses for routine vaccinations and outbreaks of diseases every year. Over the past 20 years, the organization helped provide more than 760 million children with life-saving vaccines.
Christian Schneider, Executive Director of UNICEF Germany said: “The impact of the COVID-19 pandemic threatens children around the world. Their access to health facilities is compromised, many still cannot go to school. Also, violence against children is increasing. The COVAX vaccine initiative plays a decisive role in fighting the pandemic and reducing its consequences, especially in the lower-income regions of the world. The pandemic is not over for anyone until it is over for everyone. To that end, UNICEF provides its unique and longstanding expertise in vaccine procurement and logistics. But we cannot meet this challenge alone. We therefore thank Fresenius Medical Care for their generous support.”
COVAX is a global initiative in which the vaccination alliance Gavi, the World Health Organization (WHO), UNICEF and the Coalition for Epidemic Preparedness Innovations (CEPI) are working together to facilitate the equitable global distribution of COVID-19 vaccines.
From the beginning of the COVID-19 pandemic, Fresenius Medical Care has been intensely engaged on a variety of fronts. In addition to sweeping measures to protect against infection and to ensure continuous operation of our dialysis centers, the company opened its facilities in several countries to allow vaccination of patients and, in some cases, the general public.
Fresenius Medical Care is the world's leading provider of products and services for individuals with renal diseases of which around 3.7 million patients worldwide regularly undergo dialysis treatment. Through its network of more than 4,100 dialysis clinics, Fresenius Medical Care provides dialysis treatments for approximately 346,000 patients around the globe. Fresenius Medical Care is also the leading provider of dialysis products such as dialysis machines or dialyzers. Along with its core business, the Renal Care Continuum, the company focuses on expanding in complementary areas and in the field of critical care. Fresenius Medical Care is listed on the Frankfurt Stock Exchange (FME) and on the New York Stock Exchange (FMS).
For more information visit the Company’s website at www.freseniusmedicalcare.com.
UNICEF is the United Nations Children's Fund. Every child in the world has the right to a childhood – UNICEF is there to make this right a reality. UNICEF was founded in 1946 to help children in devastated Europe after the Second World War. Today, UNICEF works in 190 countries worldwide to ensure that every child can develop healthily, grow up in a protected environment and go to school – regardless of religion, skin colour or origin.
Together with many supporters and partners, UNICEF for example provides every third child worldwide with vaccines, trains teachers, equips schools and advocates for effective child protection laws.
For more information go to: www.unicef.org
Disclaimer:
This release contains forward-looking statements that are subject to various risks and uncertainties. Actual results could differ materially from those described in these forward-looking statements due to various factors, including, but not limited to, changes in business, economic and competitive conditions, legal changes, regulatory approvals, impacts related to the COVID-19 pandemic results of clinical studies, foreign exchange rate fluctuations, uncertainties in litigation or investigative proceedings, and the availability of financing. These and other risks and uncertainties are detailed in Fresenius Medical Care AG & Co. KGaA's reports filed with the U.S. Securities and Exchange Commission. Fresenius Medical Care AG & Co. KGaA does not undertake any responsibility to update the forward-looking statements in this release.
Fresenius Medical Care, the world's leading provider of products and services for individuals with renal diseases, announced publication of its 2021 Global Annual Medical Report. Titled “Embracing the Complexity of Global Healthcare,” the report focuses on addressing healthcare complexities by driving meaningful advances in kidney disease care.
“The volume of thought leadership, ideas and expertise in the report demonstrates our company’s continued leadership in kidney care, our innovation through lifelong learning and our commitment to our patient-centered mission,” said Franklin W. Maddux, MD, Global Chief Medical Officer of Fresenius Medical Care.
The 2021 Global Annual Medical Report comprises 25 chapters by more than 40 authors from across the company. Six different sections each focus on a core theme of Fresenius Medical Care’s Clinical & Quality Agenda. An additional COVID-19 section is dedicated to the company’s ongoing efforts to address the pandemic.
The core themes are:
- Cardiovascular Health
- Precision Medicine
- Communication and Medical Education
- Global Research
- Patient-Centered Care
- Innovation and Transformation
The 2021 Global Annual Medical Report is published by the Global Medical Office of Fresenius Medical Care. The full report is now available online at: https://www.freseniusmedicalcare.com/en/about-us/sustainability/medical-responsibility/
Disclaimer:
This release contains forward-looking statements that are subject to various risks and uncertainties. Actual results could differ materially from those described in these forward-looking statements due to various factors, including, but not limited to, changes in business, economic and competitive conditions, legal changes, regulatory approvals, impacts related to the COVID-19 pandemic results of clinical studies, foreign exchange rate fluctuations, uncertainties in litigation or investigative proceedings, and the availability of financing. These and other risks and uncertainties are detailed in Fresenius Medical Care AG & Co. KGaA's reports filed with the U.S. Securities and Exchange Commission. Fresenius Medical Care AG & Co. KGaA does not undertake any responsibility to update the forward-looking statements in this release.
Fresenius Medical Care, the world's leading provider of products and services for individuals with renal diseases, is celebrating its 25th anniversary. The company, founded in 1996 when Fresenius merged its dialysis business with the U.S.-based dialysis services provider National Medical Care, now has more than 120,000 employees, operates approximately 4,100 dialysis clinics in more than 65 countries and treats about 345,000 patients. Over the past 25 years the number of patients treated in company dialysis clinics has risen more than six-fold. The production of dialysis filters (dialyzers) has increased ten-fold.
Rice Powell, CEO of Fresenius Medical Care: “Fresenius Medical Care was founded 25 years ago with the aim of continuously improving the quality of life of our patients by offering them high-quality products as well as innovative technologies and treatment concepts. We will continue to pursue these goals in the future. As the global market leader in dialysis therapies and dialysis products, we are taking every opportunity to develop further, from digitization to the development of entirely new treatment options.”
The roots of this success story go back to 1960, when Fresenius began importing dialysis machines and dialyzers from various foreign companies and distributing them in Germany, gaining a substantial market share. In 1979 the company launched its own dialysis machine, the A2008, which set a quality standard and became the world’s top seller. This role as market leader grew with the introduction of successor models from Fresenius Medical Care. Today, nearly every second dialysis machine sold worldwide is made by Fresenius Medical Care. In the early 1980s the company developed the first polysulfone dialysis filters, opening up a new era in the treatment of kidney disease. Polysulfone dialyzers are particularly effective at cleaning the patient’s blood and still set the industry standard to this day. Fresenius Medical Care has, to date, manufactured more than two billion dialyzers.
Success in dialysis products ultimately paved the way for the entry into dialysis services. In 1996, Fresenius acquired National Medical Care and combined it with its own dialysis business, a move that marked the birth of Fresenius Medical Care. That same year, the fledgling company was listed on the Frankfurt and New York exchanges that year. In 1999, Fresenius Medical Care shares gained a listing in Germany’s benchmark index, the DAX.
Since its founding, Fresenius Medical Care has been the world’s leading provider of products and services for people with renal disease. The company has steadily strengthened this position as it consistently set new milestones: In 1999, the number of dialysis machines made at company’s Schweinfurt, Germany, plant reached the 100,000 mark. In 2003, the company set two “firsts” by treating more than 100,000 patients and producing more than 50 million dialyzers that year. Total dialyzers produced reached the 500-million mark in 2007 and rose to one billion by 2013. The year before, dialysis device number 500,000 came off the Fresenius Medical Care assembly line in Schweinfurt.
Fresenius Medical Care is constantly improving dialysis technology and introducing new, innovative treatment concepts. In 2016, it launched the latest generation in hemodialysis machines, the 6008, which optimizes dialysis treatment and delivers it even more economically. In 2019, Fresenius Medical Care launched the 4008A dialysis machine developed specially to meet the needs of developing countries. The device offers Fresenius Medical Care’s highest treatment standard while keeping the cost to national health systems as low as possible.
Strong growth at Fresenius Medical Care has time and again been accompanied by large, strategic acquisitions: In 2006, the company acquired Renal Care Group, the third-largest operator of dialysis centers in the United States. That was followed, in 2011, by the purchase of Liberty Dialysis, another major U.S. dialysis center operator. With the acquisition of Euromedic that same year, Fresenius Medical Care significantly expanded its presence in Central and Eastern Europe. In 2019, the company expanded its presence in home dialysis with the acquisition of NxStage, which offered patients even more therapy options. By 2020, 12 percent of Fresenius Medical Care patients received their dialysis treatments at home.
The company expects strong growth will continue in the future. Until 2025 the company expects compounded annual growth rates in the mid-single-digit percentage range for revenue and in the high-single-digit percentage range for net income. Fresenius Medical Care believes that what until now has been very fragmented treatment of chronic kidney disease patients should be much more closely integrated through the entire course of the disease. To accomplish this, the company wants to make more effective use of core competencies in the areas of innovative products, ambulatory facilities operation, standardization of medical procedures and patient coordination.
Fresenius Medical Care has compiled a wide range of information and materials on its 25th anniversary on a special web page:
https://www.freseniusmedicalcare.com/en/25-years/
This release contains forward-looking statements that are subject to various risks and uncertainties. Actual results could differ materially from those described in these forward-looking statements due to various factors, including, but not limited to, changes in business, economic and competitive conditions, legal changes, regulatory approvals, impacts related to the COVID-19 pandemic results of clinical studies, foreign exchange rate fluctuations, uncertainties in litigation or investigative proceedings, and the availability of financing. These and other risks and uncertainties are detailed in Fresenius Medical Care AG & Co. KGaA's reports filed with the U.S. Securities and Exchange Commission. Fresenius Medical Care AG & Co. KGaA does not undertake any responsibility to update the forward-looking statements in this release.
Fresenius Medical Care expands capabilities at its Global Medical Office: As part of a holistic approach to kidney therapy, the company aims to play an active role in the field of kidney transplantation. To achieve this, Fresenius Medical Care has created the position of Head of Transplantation Medicine and appointed Dr. Benjamin Hippen (49), a US citizen, to fill the post. In this role, he will lead the company’s worldwide efforts to expand access to and understanding of transplant medicine. Dr. Hippen is a distinguished general and transplant nephrologist. He previously served on the Board of Directors of InterWell Health in North America, a nephrology network in which Fresenius Medical Care participates.
Fresenius Medical Care, the world’s leading provider of products and services for individuals with renal diseases, is investing an additional USD 25 million in the U.S. medical company Humacyte, Inc. In connection with the merger of Humacyte with a special purpose acquisition company (SPAC), Fresenius Medical Care is increasing its position in the newly combined entity as the lead investor of a private investment in public equity (PIPE). Fresenius Medical Care acquired a stake in Humacyte in 2018 for USD 150 million and agreed then on a strategic partnership. The combined company, which will be called Humacyte, is listed and trades on the Nasdaq exchange under HUMA. Fresenius Medical Care’s original stake in Humacyte will be exchanged for shares in the combined company.
Humacyte is developing implantable human acellular vessels for multiple vascular repair, reconstruction and replacement. These vessels are biologically produced blood vessels made from banked human smooth muscle cells; they are manufactured to be non-immunogenic and are to be available “off the shelf” when needed by a patient. Among numerous applications, the product is now being investigated in a clinical study for use as a vascular access for hemodialysis patients, and may prove more effective than conventional synthetic grafts and fistulas in reducing infection as well as central venous catheter use. Other possible applications include reconstruction and repair from traumatic injury, and for implants for various vascular diseases.
“Humacyte's regenerative medicine technology has great potential in many areas of healthcare,” said Franklin W. Maddux, MD, Global Chief Medical Officer of Fresenius Medical Care. “We are committed to helping Humacyte achieve market approvals with this renewed investment, to bring their technology into the mainstream of health delivery. This will bring benefits worldwide in the care of patients needing repair or reconstruction of the human vasculature. In this way, Fresenius Medical Care is consistently pursuing our strategic goal of driving medical progress with innovative therapeutic approaches, recognizing the powerful advances this represents in bringing regenerative medicine to people in need.”
Humacyte and Fresenius Medical Care had already expanded their collaboration in June: In addition to the vascular access and peripheral arterial disease applications, Fresenius Medical Care was granted exclusive rights to market the human acellular vessel outside the United States, including enhancements and modifications for vascular trauma applications.
“Our partnership with Fresenius Medical Care has developed very positively over the last three years, and we are very pleased with the company’s renewed commitment to our shared goals,” said Laura Niklason, MD, PhD, the Chief Executive Officer of Humacyte. “Our two teams have worked well to prepare for the commercialization of our human acellular vessels globally since the formation of the partnership, and we look forward to collaborating further to benefit patients and care providers, as well as the growth of our two companies.”
The biotechnology-derived blood vessel is an investigational product and is currently subject to late-stage clinical trials in the United States and Europe. Humacyte plans to file for regulatory approval in both markets after their completion Fresenius Medical Care will support the approval processes and plans the introduction in other markets worldwide with Humacyte.
Disclaimer
This release contains forward-looking statements that are subject to various risks and uncertainties. Actual results could differ materially from those described in these forward-looking statements due to various factors, including, but not limited to, changes in business, economic and competitive conditions, legal changes, regulatory approvals, impacts related to the COVID-19 pandemic results of clinical studies, foreign exchange rate fluctuations, uncertainties in litigation or investigative proceedings, and the availability of financing. These and other risks and uncertainties are detailed in Fresenius Medical Care AG & Co. KGaA's reports filed with the U.S. Securities and Exchange Commission. Fresenius Medical Care AG & Co. KGaA does not undertake any responsibility to update the forward-looking statements in this release.
Fresenius Medical Care North America opens a new laboratory in Mississippi, USA. With 200,000-square-foot (19,000 square-meters), it is the company’s largest facility of this kind. More than 300 jobs will be created over the next several years, providing the capacity to service more dialysis patients. At the new building, employees conduct comprehensive testing, analysis and reporting to ensure the best possible outcomes for patients.
Fresenius Medical Care, the world’s leading provider of products and services for individuals with renal diseases, has started using virtual reality (VR) technology to train patients to perform peritoneal dialysis. The stay•safe MyTraining VR is being offered first in Germany and will be extended to other countries in the company’s Europe, Middle East and Africa region later this year.
In peritoneal dialysis, which is used by about 11 percent of the approximately 3.7 million dialysis patients worldwide, the lining of the patient’s abdominal cavity – the peritoneum – acts as the filter for cleaning the blood. To learn how to correctly use Fresenius Medical Care’s stay•safe System for continuous ambulatory peritoneal dialysis (CAPD), home dialysis patients undergo comprehensive training in a dialysis clinic. stay•safe MyTraining VR is now supporting this training with the VR technology. The hardware comprises VR glasses and an easy-to-use controller. In various training modules, patients learn all aspects of the dialysis process, including hygiene procedures, preparation and post-therapy steps, bag-changing and operation of the stay•safe DISC.
Each training module can be adjusted to the patient’s own pace and repeated as often as needed until the patient has mastered it. While the patient trains, the nursing personnel can focus on other important tasks, for example by simultaneously assisting several other patients.
“The VR training appeals to several of the patients’ senses and this makes it especially easy to learn new procedures,” said Christoph Hame, Nursing Manager at NephroCare Germany, the service division of Fresenius Medical Care. “The VR glasses block out outside stimuli, so the patients stay focused. They can move around in the virtual room and reach for things. With this playful learning, the content is held better in the memory. Also, the standardized learning units make the training more secure, because the contents are imparted in the same way in every dialysis clinic. We offer the training in different languages, and can therefore overcome language barriers.”
The stay•safe MyTraining VR is aimed at both new and experienced home dialysis patients, who regularly update their knowledge at monthly check-ups. Patients can also be more easily included in the process of deciding which form of therapy is best for them. With the use of the VR glasses they can even learn how to handle the stay•safe System before the implantation of a catheter, which is required for the treatment.
“We want to make home dialysis possible for ever more patients,” said Dr. Katarzyna Mazur-Hofsäß, Fresenius Medical Care’s Chief Executive Officer for Europe, Middle East and Africa. “Part of this is preparing them and their family members for the challenges and changes that come along with it. With the VR training we are doing exactly that. Patients, their family members and care partners can be familiarized with the usage in a very practical, impactful way. This lowers their inhibitions and helps them with the decision on whether peritoneal dialysis at home is right for them. And it contributes to the design of home therapy that is sustainably patient-friendly and safe.”
The stay•safe MyTraining VR and the VR goggles themselves are not a medical device, but were created to support CAPD training. The training demonstrates the use of the stay-safe system. The stay•safe system for CAPD treatment includes the PIN and DISC elements and the associated peritoneal dialysis solutions.
This release contains forward-looking statements that are subject to various risks and uncertainties. Actual results could differ materially from those described in these forward-looking statements due to various factors, including, but not limited to, changes in business, economic and competitive conditions, legal changes, regulatory approvals, impacts related to the COVID-19 pandemic results of clinical studies, foreign exchange rate fluctuations, uncertainties in litigation or investigative proceedings, and the availability of financing. These and other risks and uncertainties are detailed in Fresenius Medical Care AG & Co. KGaA's reports filed with the U.S. Securities and Exchange Commission. Fresenius Medical Care AG & Co. KGaA does not undertake any responsibility to update the forward-looking statements in this release.
- As assumed, COVID-19 pandemic continued to impact organic growth in dialysis and downstream businesses
- Patient excess mortality rates significantly reduced
- Negative exchange rate effects continue
- Earnings development impacted by phasing and strong prior-year base, as indicated
- Financial targets for FY 2021 confirmed
- FME25 program on track
Rice Powell, Chief Executive Officer of Fresenius Medical Care, said: “The fact that we saw further easing in COVID-19-related excess mortality among our patients – both on a monthly basis and when looking at a rolling 12-month period – is for sure the good news we hoped for and expected to share today. We are cautious and continue to watch the Delta variant and the increasing macro-economic inflationary impacts. COVID-19 continues to impact the number of treatments in our dialysis business, the development in our downstream businesses and the speed of closing acquisitions. As indicated in May, we saw the expected significant earnings decline in the second quarter. In addition to the current effects of the pandemic, this was due to the strong prior-year base and the reversal of the favorable phasing of costs we saw in the first quarter. Based on our defined assumptions and the business performance in the first half of the year, we confirm our outlook for the full year 2021."
1 Costs related to the FME25 program
2 Net income attributable to shareholders of Fresenius Medical Care AG & Co. KGaA
COVID-19-related excess mortality declined, but continued to adversely affect the business
As expected, COVID-19 related incremental excess mortality among Fresenius Medical Care’s patients has further declined – from 3,100 patients in the first to approximately 1,500 in the second quarter. Thus, it amounted to approximately 11,200 patients in the past 12 months (as of June 30, 2021) and approximately 15,000 since the start of the pandemic. The decrease on a quarterly basis is also a result of continued progress made in patient vaccination, which has led to a further decline in global infection rates. At the end of the second quarter, approximately 71% of Fresenius Medical Care’s patients in the U.S. have received at least one dose, with a high proportion of them already fully vaccinated. On a global basis, also approximately 71% of Fresenius Medical Care’s patients have received at least one vaccination.
Fresenius Medical Care continues to monitor closely the recent COVID-19-related developments, in particular regarding the global spread of the Delta variant and any potential new waves.
As expected, COVID-19-related excess mortality of dialysis patients not only led to an underutilization of Fresenius Medical Care’s dialysis clinics, but also impacted downstream businesses in the first half of the year. Here, the U.S. Healthcare Products as well as the pharmacy business were affected by significantly lower than expected volumes.
The anticipated decline in incremental excess mortality has led to a smaller impact in Q2 than in Q1. The overall adverse COVID-19 impact from accumulated excess mortality on organic growth in the Health Care Services business amounted to around 240 basis points in the second quarter and 290 basis points in the first half of 2021.
Outlook
Based on the results for the second quarter and first half, Fresenius Medical Care confirms its outlook for FY 2021 as outlined in February. The Company expects revenue to grow at a low- to mid-single digit percentage rate and net income to decline at a high-teens to mid-twenties percentage rate against the 2020 base. This outlook is based on the assumption of a return to normalized mortality rates in the second half of 2021.
To support its 2025 strategy, further strengthen profitability and compensate for the negative earnings effects of the COVID-19 pandemic, Fresenius Medical Care has initiated the FME25 program. The Company reconfirms its targets and is on track with its work regarding the operating model transformation and efficiency measures. Fresenius Medical Care will provide an update in the fall.
3 These targets are based on the 2020 results excluding the impairment of goodwill and trade names in the Latin America Segment of EUR 195 million. They are inclusive of anticipated COVID-19 effects, in constant currency and exclude special items. Special items include costs related to FME25 and other effects that are unusual in nature and have not been foreseeable or not foreseeable in size or impact at the time of giving guidance.
Driving sustainability efforts
Sustainability is an integral part of Fresenius Medical Care’s mission and vision and is reflected in the corporate strategy. The Company is committed to implementing global sustainability standards in its operations around the world. To this end, Fresenius Medical Care is further driving forward its Global Sustainability Program and ESG initiatives:
Fresenius Medical Care has recently joined econsense, a network of companies united in the goal of shaping the transformation to a sustainable economy and society. The dialogue and cross-industry exchange of practical expertise within econsense, which now counts 40 major companies as members, will further support Fresenius Medical Care’s sustainability management.
In July, Fresenius Medical Care has further underlined its commitment to create value in ecological, social and economic terms by signing its first sustainability-linked financing instrument. The Company’s new EUR 2 billion syndicated revolving credit facility includes a component that links its margin development to sustainability performance.
Earnings impacted by COVID-19, negative exchange rate effects and a high prior-year base
Revenue in the second quarter decreased by 5% to EUR 4,320 million (+2% at constant currency, +1% organic).
Health Care Services revenue in the second quarter declined by 6% to EUR 3,400 million (+2% at constant currency, +1% organic). The increase in organic growth in the international regions was more than offset by negative exchange rate effects in all regions as well as by the adverse COVID-19 impact and a lower reimbursement for Calcimimetics in North America.
Health Care Products revenue decreased by 2% to EUR 920 million (+2% at constant currency, +1% organic). The positive organic growth development was mainly driven by higher sales of in-center disposables in EMEA and Asia-Pacific, machines for chronic treatment and renal pharmaceuticals. This positive development was offset mainly by negative exchange rate effects and lower sales of acute care products compared to a strong prior-year base.
In the first half, revenue declined by 6% to EUR 8,530 million (+2% at constant currency, +1% organic). Health Care Services revenue decreased by 7% to EUR 6,726 million (+1% at constant currency, +1% organic); Health Care products revenue declined by 2% to EUR 1,804 million (+3% at constant currency, +3% organic).
Operating income decreased by 35% to EUR 424 million in the second quarter (-30% at constant currency), resulting in a margin of 9.8% (Q2 2020: 14.4%). Operating income excluding special items declined by 34% to EUR 430 million (-29% at constant currency), resulting in a margin of 10.0%. The decrease was mainly due to the adverse impact of the COVID-19 pandemic, including a high prior-year base as a result of government relief funding, the expected phasing and increase in Sales, General and Administrative expense, negative exchange rate effects and higher direct costs. These effects were partially offset in particular by an improved Medicare Advantage payor mix in the U.S.
In the first half, operating income decreased by 26% to EUR 898 million (-20% at constant currency), resulting in a margin of 10.5% (H1 2020: 13.4%). Operating income excluding special items declined by 25% to EUR 907 million (-19% at constant currency), resulting in a margin of 10.6%.
Net income2 decreased by 38% to EUR 219 million in the second quarter (-33% at constant currency). Net income excluding special items declined by 37% to EUR 223 million (-31% at constant currency).
In the first half, net income decreased by 26% to EUR 468 million (-21% at constant currency). Net income excluding special items declined by 25% to EUR 474 million (-20% at constant currency).
Basic earnings per share (EPS) decreased by 38% to EUR 0.75 (-33% at constant currency) in the second quarter. EPS excluding special items declined by 37% to EUR 0.76 (-31% at constant currency).
In the first half, EPS decreased by 26% to EUR 1.60 (-20% at constant currency). EPS excluding special items declined by 25% to EUR 1.62 (-19% at constant currency).
Cash flow development
In the second quarter, Fresenius Medical Care generated EUR 921 million of operating cash flow (Q2 2020: EUR 2,319 million), resulting in a margin of 21.3% (Q2 2020: 50.9%). The decline was mainly due to the U.S. federal government’s payments in the second quarter of 2020 under the CARES Act, the start of recoupment of these advanced payments in the second quarter of 2021 as well as the timing of certain other expense payments in 2021. In the first half, operating cash flow amounted to EUR 1,129 million (H1 2020: EUR 2,903 million), resulting in a margin of 13.2% (H1 2020: 32.1%).
Fresenius Medical Care generated EUR 720 million of free cash flow4 (Q2 2020: EUR 2,103 million) in the second quarter, resulting in a margin of 16.7% (Q2 2020: 46.1%). In the first half, Free cash flow amounted to EUR 749 million (H1 2020: EUR 2,407 million), resulting in a margin of 8.8% (H1 2020: 26.6%).
4 Net cash provided by / used in operating activities, after capital expenditures, before acquisitions, investments and dividends
Regional developments
In North America, revenue decreased by 9% to EUR 2,953 million in the second quarter (stable at constant currency, -1% organic). Besides a sizable negative exchange rate effect, this was mainly due to the adverse COVID-19 impact on both the Health Care Services and Health Care Products businesses along with associated downstream effects and a lower reimbursement for Calcimimetics. In the first half, revenue decreased by 9% to EUR 5,852 million (stable at constant currency, -1% organic).
Operating income in North America decreased by 35% to EUR 398 million in the second quarter (-29% at constant currency), resulting in a margin of 13.5% (Q2 2020: 18.8%). The decline was mainly due to the adverse impact of COVID-19, including a high prior-year base mainly driven by government relief funding, negative exchange rate effects, increased direct costs and an unfavorable impact from Calcimimetics. This was partially offset by an improved Medicare Advantage payor mix. In the first half, operating income declined by 26% to EUR 796 million (-19% at constant currency), resulting in a margin of 13.6% (H1 2020: 16.7%).
Revenue in EMEA increased by 1% and amounted to EUR 693 million in the second quarter (+2% at constant currency, +2% organic). This was mainly driven by an increase in Health Care Product revenue due to higher sales of acute cardiopulmonary products, renal pharmaceuticals and machines for chronic treatment, partially offset by lower sales of products for acute treatments and negative exchange rate effects. In the first half, revenue was stable and amounted to EUR 1,362 million (+2% at constant currency, +2% organic).
Operating income in the EMEA region decreased by 5% to EUR 73 million in the second quarter (-5% at constant currency), resulting in a margin of 10.6% (Q2 2020: 11.3%). The decline was mainly driven by negative foreign currency transaction effects, higher costs of revenue as well as higher Sales, General and Administrative expense. This was partially offset by the lower prior year base due to an impairment recorded in 2020 for a license held by the joint venture with Vifor Pharma. In the first half, operating income decreased by 14% to EUR 153 million (-14% at constant currency), resulting in a margin of 11.2% (H1 2020: 13.1%).
In Asia-Pacific, revenue increased by 8% to EUR 486 million in the second quarter (+11% at constant currency, +10% organic). This was mainly driven by an increase in Health Care Services revenue, primarily due to a recovery in elective procedures, which was partially offset by exchange rate effects. In the first half, revenue grew by 7% to EUR 957 million (+11% at constant currency, +11% organic).
Operating income increased by 33% to EUR 84 million in the second quarter (+38% at constant currency), resulting in a margin of 17.3% (Q2 2020: 14.1%). The increase was mainly driven by favorable business growth and the above-mentioned recovery in elective procedures, partially offset by unfavorable foreign currency transaction effects. In the first half, operating income grew by 21% to EUR 170 million (+25% at constant currency), resulting in a margin of 17.7% (H1 2020: 15.7%).
Despite a very significant headwind from exchange rates and the adverse impact of COVID-19, Latin America revenue increased by 1% to EUR 171 million in the second quarter (+17% at constant currency, +14% organic). This was mainly driven by an increase in Health Care Services revenue. In the first half, revenue decreased by 2% to EUR 330 million (+17% at constant currency, +15% organic).
Operating income in Latin America decreased by 76% to EUR 3 million in the second quarter (-82% at constant currency), resulting in a margin of 1.5% (Q2: 2020: 6.4%). The decline was driven by increased bad debt expense in Colombia. In the first half, operating income declined by 48% to EUR 9 million (-49% at constant currency), resulting in a margin of 2.8% (H1 2020: 5.3%).
Patients, Clinics and Employees
As of June 30, 2021, Fresenius Medical Care treated 345,646 patients in 4,125 dialysis clinics worldwide. At the end of the second quarter, the Company had 123,538 employees (full-time equivalents) worldwide, compared to 124,736 employees as of June 30, 2020.
Conference call
Fresenius Medical Care will host a conference call to discuss the results of the second quarter and first half of 2021 on July 30, 2021 at 3:30 p.m. CEST / 9:30 a.m. EDT. Details will be available on the company’s website www.freseniusmedicalcare.com in the “Investors” section. A replay will be available shortly after the call.
Please refer to our statement of earnings included in the PDF-file for a complete overview of the results of the second quarter and first half of 2021. Our 6-K disclosure provides more details.
This release contains forward-looking statements that are subject to various risks and uncertainties. Actual results could differ materially from those described in these forward-looking statements due to various factors, including, but not limited to, changes in business, economic and competitive conditions, legal changes, regulatory approvals, impacts related to the COVID-19 pandemic results of clinical studies, foreign exchange rate fluctuations, uncertainties in litigation or investigative proceedings, and the availability of financing. These and other risks and uncertainties are detailed in Fresenius Medical Care AG & Co. KGaA's reports filed with the U.S. Securities and Exchange Commission. Fresenius Medical Care AG & Co. KGaA does not undertake any responsibility to update the forward-looking statements in this release.