Fresenius Medical Care, the world’s largest provider of dialysis products and services, expects continued strong growth. At the Annual General Meeting in Frankfurt today, CEO Rice Powell explained the company's growth strategy for the coming years: “We will continue to expand our business with services and products for dialysis, and will continue to grow,” Powell said in his speech to the shareholders. “The basis for this is our international network of dialysis centers, our comprehensive knowledge in dialysis, and focusing our Care Coordination portfolio. We are well positioned to respond to the current and future changes in health care systems: In fact, we can actively shape these systems! This helps our patients, because we can provide them with comprehensive care. And in turn, helping our patients is the key to our business success.”
A large shareholder majority of 88.27 percent approved a 10 percent increase in the dividend, from €0.96 to €1.06, the company’s 21st consecutive dividend increase. Shareholder majorities of 99.23 and 95.46 percent, respectively, approved the actions of the Management and Supervisory Boards in 2017.
At the Annual General Meeting, 80.46 percent of the subscribed capital was represented.
Dr. Gerd Krick, Chairman of the Supervisory Board of Fresenius Medical Care AG & Co. KGaA, announced that he will resign from the Supervisory Board, effective at the meeting’s end. He will maintain his position on the Supervisory Board of the General Partner, Fresenius Medical Care Management AG.
Dr. Krick had been Chief Executive Officer of Fresenius Medical Care from 1996, when the company was founded, to 1998, laying the foundation for its global success. In 1998, he resigned from his CEO position and became Chairman of the Supervisory Board of Fresenius Medical Care. The Supervisory Board and Management Board thank Dr. Krick for his tremendous efforts to date and many years of service to the benefit of the company.
The Supervisory Board has appointed Dr. Dieter Schenk, Vice Chairman of the Supervisory Board of Fresenius Medical Care AG & Co. KGaA, as the new Chairman. A new member of the Supervisory Board will be appointed in due course and proposed for election at the next Annual General Meeting, which is expected to take place on May 16, 2019.
Disclaimer
This release contains forward-looking statements that are subject to various risks and uncertainties. Actual results could differ materially from those described in these forward-looking statements due to certain factors, including changes in business, economic and competitive conditions, regulatory reforms, foreign exchange rate fluctuations, uncertainties in litigation or investigative proceedings, and the availability of financing. These and other risks and uncertainties are detailed in Fresenius Medical Care AG & Co. KGaA's reports filed with the U.S. Securities and Exchange Commission. Fresenius Medical Care AG & Co. KGaA does not undertake any responsibility to update the forward-looking statements in this release.
- Revenue and net income growth in the first quarter impacted by significant currency headwind
- Growth delivered by Products business and International segments
- On track to achieve net income growth target
- Expected revenue growth softened due to reduced dosing of calcimimetic drugs in the United States
- Divestiture of Sound Inpatient Physicians to further focus U.S. Care Coordination activities

“On the back of a solid first quarter, which showed healthy organic growth in our businesses, we are heading towards another record year in our company’s history. While managing the shift of the calcimimetic drugs into our clinical operations in the United States, we achieved good organic revenue growth in our Dialysis Services business and strong organic revenue growth in our Products business. This provides a solid basis to deliver on our growth targets for this year. With the planned sale of Sound Inpatient Physicians we have narrowed the focus of our Care Coordination strategy in the U.S. on areas that provide the highest contribution and the best outcomes for our patients,” said Rice Powell, Chief Executive Officer of Fresenius Medical Care. “I am proud to announce that we have just proven once again our commitment to patients. We have received the highest quality rankings in the industry from the U.S. Centers for Medicare and Medicaid Services last week.”
Currency headwind impacts revenue and earnings, healthy organic growth continues
Revenue in the first quarter 2018 was significantly impacted by a 12% negative impact resulting from foreign currency translation, declining 1% at constant currency to EUR 3,976 million. Adjusting Q1 2017 for the prior year impact from the recognition of revenue related to the agreement with the U.S. Departments of Veterans Affairs and Justice (VA Agreement) and for the IFRS 15 implementation, revenue growth in the first quarter 2018 was 4% at constant currency. Health Care Services revenue declined by 3% at constant currency (EUR 3,209 million), driven by the effect of the implementation of IFRS 15. Health Care Products revenue increased by 6% at constant currency to EUR 767 million. Organic growth for Health Care Services was at 2%, and for the Health Care Products business at 6%. Dialysis treatments increased by 3% as a result of growth in same-market treatments (2%) and contributions from acquisitions (1%).
Total operating income (EBIT) reached EUR 497 million (margin of 12.5%, 180 basis points below the level of last year). This development was strongly impacted by the VA Agreement and the impact from the initial increase in valuation of Sound Physicians’ share based payment program caused by the sale of Sound Physician (initial Sound valuation impact). Adjusting for the revenue impact from the implementation of IFRS 15 and excluding the VA Agreement as well as the initial Sound valuation impact, EBIT grew by 3% at constant currency and EBIT margin was stable at 12.8%.
Net income attributable to shareholders of Fresenius Medical Care AG & Co. KGaA remained strong with EUR 279 million, a stable development at constant currency. Adjusted for the negative impact of the initial Sound valuation impact net income grew by 5% on a constant currency basis (EUR 292 million). Excluding all special items – namely the VA Agreement in Q1 2017 and the positive effect from the U.S. Tax Reform in Q1 2018 as well as the initial Sound valuation impact, net income attributable to shareholders of Fresenius Medical Care AG & Co. KGaA increased by 8% at constant currency.
Based on the number of approximately 306.5 million shares (weighted average number of shares outstanding), basic earnings per share (EPS) amounted to EUR 0.91, compared to EUR 1.01 for the first quarter of 2017.
Development of Reporting Segments
North America revenue, which corresponds to 70% of total revenue, was down by 5% at constant currency to EUR 2,774 million.
Dialysis Care revenue decreased by 16% to EUR 2,075 million, including a 13% negative impact resulting from foreign currency translation. At constant currency, Dialysis Care revenue decreased by 3%, mainly due to the implementation of IFRS 15 (EUR 88 million) and the prior-year impact from the VA Agreement (EUR 100 million). Excluding the 2017 effect from the VA Agreement and the 2017 effect from the implementation of IFRS 15, Dialysis Care revenue increased by 5% at constant currency. Same-market treatments grew by 2%, organic revenue per treatment by 2% and acquisitions contributed 1%. At constant currency, Care Coordination revenue decreased by 14%, driven by the shift of calcimimetic drugs into the clinical environment, the implementation of IFRS 15 and the impact from the Shiel Laboratories divestiture in Q4 2017.
As of the end of March 2018, the company was treating 197,339 patients at its 2,419 clinics in North America. Both numbers increased by 4%, while dialysis treatments increased by 3%.


In the U.S., the average revenue per treatment, adjusted for the implementation of IFRS 15 and excluding the 2017 impact of the VA Agreement, the average revenue per treatment increased by USD 6 from USD 342 to USD 348. The increase was mainly driven by the announced initial introduction of calcimimetic drugs in the clinical environment, which is still in an early conversion stage. Higher implicit price concessions (IFRS 15) and, as previously indicated, lower revenue from commercial payors mitigated this effect.
Cost per treatment in the U.S., adjusted for the implementation of IFRS 15, increased to USD 288 (from USD 276). This development was largely a result of the announced initial introduction of calcimimetic drugs in the clinical environment, which is still in an early conversion stage, higher personnel expense, as well as increased property and other occupancy related costs and increased costs for medical supplies partially offset by lower costs for health care supplies.
At constant currency, Health Care Products revenue increased by 1% due to higher sales of renal pharmaceuticals, peritoneal dialysis products, hemodialysis solutions and concentrates, partially offset by lower sales of machines and dialyzers.
The total operating income of the North America segment was EUR 362 million (-31%), (-21% at constant currency), an operating income margin of 13.1%. Adjusted for the initial Sound valuation impact and the 2017 effects from the VA Agreement, operating income (EBIT) was EUR 375 million compared to EUR 427 million in the first quarter 2017. The respective operating income margin was 13.5% compared to 13.6% in the first quarter of 2017.
EMEA revenue increased by 6% at constant currency to EUR 636 million, mainly driven by positive business development in Health Care Services revenue and Health Care Products revenue, which both increased by 6% at constant currency. The increase in Health Care Services revenue was driven by acquisitions, same-market treatment growth and an increase in dialysis days. Dialysis Products revenue grew by 7% at constant currency to EUR 302 million, due to higher sales of products for acute care treatments, machines, peritoneal dialysis products and renal pharmaceuticals.
Non-dialysis Products revenue decreased by 6% to EUR 20 million, primarily due to lower sales of acute cardiopulmonary products. There was virtually no impact from foreign currency translation effects. Operating income was EUR 109 million. The operating income margin decreased from 18.7% to 17.1%, mainly due to unfavorable impacts from currency effects partially offset by the impact of one additional dialysis day.
As of the end of March 2018, the company had 63,114 patients (5% increase) being treated at 754 clinics (4% increase) in the EMEA region. Dialysis treatments increased by 5%.
Asia-Pacific revenue grew by 14% at constant currency to EUR 392 million. In the region, Health Care Services revenue increased 9% (20% at constant currency) to EUR 184 million. Care Coordination activities contributed EUR 46 million to Health Care Services revenue. With growth of 8% in constant currency to EUR 208 million, the Health Care Products business showed a solid sales performance, mainly driven by higher sales of chronic hemodialysis products and products for acute care treatments. Operating income reached EUR 74 million (Q1 2017: EUR 82 million). The operating income margin decreased to 19.0% in Q1 2018 (Q1 2017: 21.7%). This was primarily driven by foreign currency transaction effects and delayed product sales.
As of the end of March 2018, the company had 30,194 patients (2% increase) being treated at 385 clinics in Asia-Pacific. Dialysis treatments increased by 2%, impacted by same market treatment growth of 4% partially offset by the effect of sold or closed clinics.
Latin America delivered revenue of EUR 170 million, a significant improvement of 17% at constant currency. This growth was mainly driven by an increase in organic revenue per treatment. Health Care Products revenue grew by 25% at constant currency based on higher sales of dialyzers, machines, products for acute treatments and peritoneal dialysis products. With an operating income of EUR 14 million the segment generated an operating income on previous year’s level. Operating income margin increased slightly to 8.3% in Q1 2018 (Q1 2017: 8.1%).
As of the end of March 2018, the company was treating 31,606 patients (5% increase) at 232 clinics in Latin America. Dialysis treatments increased by 4%.
Net interest expense was EUR 80 million compared to EUR 92 million in the first quarter of 2017, a decrease of 14% (5% at constant currency). The decrease was driven by the lower leverage level and a repayment of high interest-bearing senior notes. Income tax expense was EUR 87 million for the first quarter of 2018, which translates into an effective tax rate of 20.9%, compared to last year’s Q1 with a tax rate of 32.5%. The strong reduction was largely driven by the U.S. Tax Reform.
Cash flow with normalized development
In the first quarter of 2018, the company used EUR 45 million in net cash from operating activities, compared to EUR 170 million provided by operating activities in last year’s Q1. The decrease in net cash provided by operating activities was largely driven by the positive impact from the 2017 cash inflow related to the VA Agreement, a higher impact from seasonality in invoicing and increased inventory levels, partially offset by a positive impact from lower income tax payments. The number of days sales outstanding (DSOs) increased sequentially by 10 days compared with Q4 2017 to reach 85 days driven by the above mentioned seasonality in invoicing. Free cash flow (Net cash used in operating activities, after capital expenditures, before acquisitions and investments) amounted to EUR (263 million) and EUR (25 million) for the three months ended March 31, 2018 and March 31, 2017, respectively. Free cash flow in percent of revenue was (6.6%) and (0.6%) for the three months ended 2018 and 2017, respectively.
Focusing the profile in U.S. Care Coordination portfolio
On April 21, Fresenius Medical Care announced to divest its controlling interest in Sound Inpatient Physicians to an investment consortium for total transaction proceeds of USD 2.15 billion (EUR 1.76 billion3). The divestment is expected to generate a pre-tax book gain of approximately EUR 800 million3,4. Closing of the transaction is subject to regulatory approvals and anticipated late in 2018.
Employees
As of March 31, 2018, Fresenius Medical Care had 114,831 employees (full-time equivalents) worldwide, compared to 110,530 employees at the end of March 2017. This increase was mainly attributable to our continued organic growth and acquisitions.
Outlook 2018
The company expects revenue5 growth between 5% and 7% at constant currency. Adjusted net income6 is expected to increase by 13% to 15% at constant currency and excluding special items7 to increase by 7% to 9%.
The targets exclude effects from major transactions such as the planned acquisition of NxStage Medical and the planned divestiture of Sound Physicians.
Conference call
Fresenius Medical Care will host a conference call to discuss the results of the first quarter today at 3:30 p.m. CEDT / 9:30 a.m. EDT. Details will be available on the company’s website www.freseniusmedicalcare.com in the “Investors/Events” section. A replay will be available shortly after the call.
1For a detailed reconciliation, please refer to the table at the end of the press release
2Net income attributable to shareholders of Fresenius Medical Care AG & Co. KGaA
3EUR/USD 1.22
4Based on the company’s latest available valuation of the North American operating segment
52017 adjusted for the effect of IFRS 15 implementation
6Attributable to shareholders of Fresenius Medical Care AG & Co. KGaA, adjusted for the Sound valuation impact
7VA Agreement, Natural Disaster Costs, FCPA related charge, U.S. Tax Reform
Disclaimer
This release contains forward-looking statements that are subject to various risks and uncertainties. Actual results could differ materially from those described in these forward-looking statements due to certain factors, including changes in business, economic and competitive conditions, regulatory reforms, foreign exchange rate fluctuations, uncertainties in litigation or investigative proceedings, and the availability of financing. These and other risks and uncertainties are detailed in Fresenius Medical Care AG & Co. KGaA's reports filed with the U.S. Securities and Exchange Commission. Fresenius Medical Care AG & Co. KGaA does not undertake any responsibility to update the forward-looking statements in this release.
Fresenius Medical Care, the world’s largest provider of dialysis products and services, today announced a leadership change in its EMEA (Europe, Middle East and Africa) region. Effective 1 September 2018, Ms. Katarzyna Mazur-Hofsäss, Ph.D., will assume the Management Board position in charge of EMEA. She follows Dominik Wehner, who decided to step down from his position for personal reasons, effective on 31 December 2017. In the interim period Rice Powell, Chief Executive Officer of Fresenius Medical Care and Chairman of the Management Board, manages the EMEA region.
“I want to thank Dominik for his more than 20 years of contributions – most notably his commitment of caring for our patients at all times while enhancing our geographical footprint in the EMEA region. We are very proud of what Dominik has achieved and wish him all the best for his future,” said Rice Powell.
Katarzyna Mazur-Hofsäss has been president for EMEA at the med-tech company Zimmer Biomet since 2013. In her 25 years of professional career she has gained extensive experience in the medical and pharma industry from her positions at Abbott Laboratories and Roche. Katarzyna Mazur-Hofsäss is a physician by educational background and holds a Ph.D. from Gdansk Medical University in Poland, as well as an MBA from the Warsaw School of Economics and the University of Minnesota. “Katarzyna will not only be well equipped to run our expanding EMEA region but she will also have a fresh perspective that can provide new impulses. We look forward to welcoming her in our team,” said Rice Powell.
Disclaimer
This release contains forward-looking statements that are subject to various risks and uncertainties. Actual results could differ materially from those described in these forward-looking statements due to certain factors, including changes in business, economic and competitive conditions, regulatory reforms, foreign exchange rate fluctuations, uncertainties in litigation or investigative proceedings, and the availability of financing. These and other risks and uncertainties are detailed in Fresenius Medical Care AG & Co. KGaA's reports filed with the U.S. Securities and Exchange Commission. Fresenius Medical Care AG & Co. KGaA does not undertake any responsibility to update the forward-looking statements in this release.
Fresenius Medical Care, the world’s largest provider of dialysis products and services, has taken another step in the expansion of its production and development site in Schweinfurt, Germany. At an official ceremony today attended by Schweinfurt Mayor Sebastian Remelé, the company broke ground on a new technology center for developing dialysis machines.
As many as 250 employees from different departments will be able to work under the same roof in the 8,000-square-meter (86,000-square-foot) building. Using an open concept and designed to minimize distances that need to be covered, it will facilitate cooperation and exchanges between different teams. Fresenius Medical Care is investing a double-digit million euro sum in the construction, which is scheduled for completion late next year.
“With the new technology center, we are aiming to mesh production and development much closer together,” said Kent Wanzek, Fresenius Medical Care’s Chief Executive Officer for Global Manufacturing and Quality. “This will enable us to do an even better job developing high-quality yet affordable dialysis products for a steadily increasing number of patients.”
Dr. Olaf Schermeier, Fresenius Medical Care’s Chief Executive Officer for Global Research and Development, said: “The innovative therapy systems that will originate in our new technology center will improve the lives of our patients worldwide.”
Mayor Remelé said: “Fresenius Medical Care has been a major employer in this region and a pillar of Schweinfurt’s economy for many years. We see the building of the new technology center as a real commitment to this city, and we’re very happy about it.”
The plant in Schweinfurt, which was established in 1979, is Fresenius Medical Care’s largest development and production facility for dialysis machines and other medical devices. The company now employs more than 1,200 people in the city, about 120 kilometers (75 miles) east of Frankfurt in northern Bavaria. About one third of them work in research and development.
Dialysis machines, bloodline systems and dialyzers – the latter often dubbed “artificial kidneys” because this is where the blood is actually cleaned – are the most important products for treating chronic kidney disease. During treatment, the dialysis machine pumps the patient’s blood through bloodlines, monitors its circulation through the dialyzer, and adds anti-coagulants. Treatments are generally carried out three times weekly, and take between three and six hours each.
This release contains forward-looking statements that are subject to various risks and uncertainties. Actual results could differ materially from those described in these forward-looking statements due to certain factors, including changes in business, economic and competitive conditions, regulatory reforms, foreign exchange rate fluctuations, uncertainties in litigation or investigative proceedings, and the availability of financing. These and other risks and uncertainties are detailed in Fresenius Medical Care AG & Co. KGaA's reports filed with the U.S. Securities and Exchange Commission. Fresenius Medical Care AG & Co. KGaA does not undertake any responsibility to update the forward-looking statements in this release.
- Adjusted revenue growth target in 2018 of 5 to 7% at constant currency expected (previously around 8%) mainly due to recent reduction in dosing of calcimimetic drugs
- Reconfirmed reported net income growth target of 13 to 15% at constant currency in 2018
- Preliminary indicative first quarter 2018 results impacted by strong currency headwind and positive one-time effect in previous year
“First quarter results are impacted by a shift of calcimimetic drugs from our pharmacy business into the dialysis service business in the U.S. Due to a faster than expected reduction in dosing of those drugs in the controlled clinic environment, we are experiencing a headwind on revenue growth for fiscal 2018,” said Rice Powell, CEO of Fresenius Medical Care. “Based on our solid underlying business and the planned phasing of net income growth we confirm our net income growth target for 2018.”
Clinical introduction of calcimimetic drugs with impact on revenue growth 2018
The addition of Parsabiv as an injectable calcimimetic drug in the U.S. triggered the move of the corresponding reimbursement for Medicare patients from Part D to Part B. This resulted in a revenue decline in the Company´s pharmacy business and a lower than assumed revenue increase in the dialysis service business driven by lower than expected dosing of calcimimetic drugs. It is imperative that drugs are delivered in an efficient and effective manner in Fresenius Medical Care´s controlled clinic environment to the patients who benefit from the use of those drugs. The Company actively manages all classes of medications focusing on the quality outcomes for patients and the overall cost to the respective health care system.
Preliminary indicative key figures (IFRS) – first quarter 2018 compared to first quarter 2017, adjusted for IFRS 15
| EUR million | Q1 2018 | Growth yoy | Growth yoy at cc |
| Revenue | 3,976 | (10%) | 2% |
| Revenue Q1 2017 excluding VA Agreement1 | 3,976 | (8%) | 4% |
| Operating income (EBIT) | 497 | (24%) | (15%) |
| Operating income (EBIT adjusted for valuation of the Sound Physicians' share based payments2 and excluding VA Agreement Q1 20171 |
510 510 |
(22%) (8%) |
(13% 3% |
| Net income3 | 279 | (10%) | 0% |
| Net income adjusted for valuation of the Sound Physicians' share based payments2 and excluding special items (VA Agreement1, U.S. Tax Reform) |
292 244 |
(5%) (2%) |
5% 8% |
For a detailed reconciliation, please refer to the table at the end of the press release.
1Agreement with the United States Departments of Veterans Affairs and Justice
2Initial increase in valuation of the Sound Physicians’ share based payment program caused by sale of Sound Physicians
3Net income attributable to shareholders of Fresenius Medical Care AG & Co. KGaA
Preliminary first quarter 2018 results
Revenue in the first quarter 2018 was strongly impacted by headwinds from foreign exchange rates, the expected decline in our North American Care Coordination business and the positive one-time effect in Q1 2017. Revenue came in below the level of the previous year’s quarter with EUR 3,976 million. Adjusted for IFRS 15 and at constant currency, revenue increased by 2%, excluding the VA Agreement in Q1 2017 the first quarter growth was 4% at constant currency.
Operating income (EBIT) in the first quarter 2018 reached EUR 497 million. Adjusted for the first quarter effect of the valuation of Sound Physicians’ share based payment program in connection with the divestiture of Sound Physicians and for the positive impact of the VA Agreement in Q1 2017 EBIT was up by 3% at constant currency.
Net income1 for the first quarter of 2018 was stable at constant currency at EUR 279 million. Adjusted for the first quarter effect of the valuation of Sound Physicians’ share based payment program and the effect of the U.S. Tax Reform in 2018 and for the positive impact of the VA Agreement in Q1 2017, net income was 8% ahead in constant currency of last year’s first quarter.
1Net income attributable to shareholders of Fresenius Medical Care AG & Co. KGaA
Outlook 2018
Mainly driven by the change in calcimimetic drugs Fresenius Medical Care expects reduced revenue growth of 5 to 7% (previously: around 8%) at constant currency. The company continues to expect reported net income growth of 13 to 15% at constant currency.
The targets are based on 2017 adjusted for the effect of the IFRS 15 implementation and exclude effects from major transactions such as the planned acquisition of NxStage Medical and the sale of Sound Physicians.
Publication and conference call
Fresenius Medical Care will publish the results of the first quarter, as scheduled, on Thursday, May 3, at 7:00 a.m. CET and hold a conference call to discuss the results of the first quarter at 3:30 p.m. CET / 9:30 a.m. EDT. Details will be available on the company’s website www.freseniusmedicalcare.com in the “Investors/Events” section. A replay will be available shortly after the call.
Fresenius Medical Care is the world's largest provider of products and services for individuals with renal diseases of which around 3.2 million patients worldwide regularly undergo dialysis treatment. Through its network of 3,752 dialysis clinics, Fresenius Medical Care provides dialysis treatments for 320,960 patients around the globe. Fresenius Medical Care is also the leading provider of dialysis products such as dialysis machines or dialyzers. Along with the core business, the company focuses on expanding the range of related medical services in the field of Care Coordination. Fresenius Medical Care is listed on the Frankfurt Stock Exchange (FME) and on the New York Stock Exchange (FMS).
For more information visit the Company’s website at www.freseniusmedicalcare.com.
Disclaimers
This release contains forward-looking statements that are subject to various risks and uncertainties. Actual results could differ materially from those described in these forward-looking statements due to certain factors, including changes in business, economic and competitive conditions, regulatory reforms, foreign exchange rate fluctuations, uncertainties in litigation or investigative proceedings, and the availability of financing. These and other risks and uncertainties are detailed in Fresenius Medical Care AG & Co. KGaA's reports filed with the U.S. Securities and Exchange Commission. Fresenius Medical Care AG & Co. KGaA does not undertake any responsibility to update the forward-looking statements in this release.
Fresenius Medical Care, the world’s largest provider of dialysis products and services, today announced an important strategic step to sharpen its U.S. Care Coordination profile with the signing of a definitive agreement to divest its controlling interest in Sound Inpatient Physicians Holdings, LLC (Sound) to an investment consortium led by Summit Partners (Summit), for total transaction proceeds of $2.15 billion (EUR 1.76 billion1). Sound is a physician organization providing services across the acute episode of care – through emergency medicine, critical care, hospital medicine, transitional care and advisory services (www.soundphysicians.com).
To gain a deeper understanding and expertise on the highly efficient coordination of patients while improving the quality outcomes and reducing total cost to the health care system at the same time, Fresenius Medical Care invested to become majority shareholder in Sound in 2014. In the same year, Sound expanded its footprint with the acquisition of Cogent Healthcare, Inc. Due to the participation in the major value-based program BPCI (Bundled Payments for Care Improvement) focusing on improving quality while lowering costs, Fresenius Medical Care was able to learn and significantly broaden its experience in value-based care programs in the U.S.
In 2017, Sound generated revenues of around EUR 1.25 billion and EBIT of around EUR 90 million with 3,500 employees.
“In the previous years we have gained insight and successfully applied the knowledge on efficient patient coordination and on value-based programs in our unit called Fresenius Health Partners where we run the ESCOs, our own Medicare Advantage plan and various sub-capitated arrangements. This was an important milestone in the execution of our Care Coordination strategy,” said Rice Powell, Chief Executive Officer of Fresenius Medical Care. “As we are now very well positioned in the U.S. to ensure high quality outcomes for our dialysis patients in a health care system that is moving towards value-based care, we are enabled to divest Sound and release the invested capital for further focused growth investments to add value for our shareholders.”
“The acquisition by Summit provides Sound with the opportunity to expand its existing service lines in a rapidly changing market and tap into new areas. This is a great chance for Sound’s employees to be part of this next development step,” said Bill Valle, Chief Executive Officer of Fresenius Medical Care North America.
The divestment is expected to generate a pre-tax book gain for Fresenius Medical Care of approximately EUR 800 million1,2. Closing of the transaction is subject to regulatory approvals and anticipated late in 2018.
The financial targets for 2018 and 2020 do not include the effects of this divesture or the planned acquisition of NxStage Medical, Inc.
1 EUR/USD 1.22
2 Based on the company’s latest available valuation of the North American business segment
Fresenius Medical Care is the world's largest provider of products and services for individuals with renal diseases of which around 3.2 million patients worldwide regularly undergo dialysis treatment. Through its network of 3,752 dialysis clinics, Fresenius Medical Care provides dialysis treatments for 320,960 patients around the globe. Fresenius Medical Care is also the leading provider of dialysis products such as dialysis machines or dialyzers. Along with the core business, the company focuses on expanding the range of related medical services in the field of Care Coordination. Fresenius Medical Care is listed on the Frankfurt Stock Exchange (FME) and on the New York Stock Exchange (FMS).
For more information visit the company’s website at www.freseniusmedicalcare.com.
Disclaimer
This release contains forward-looking statements that are subject to various risks and uncertainties. Actual results could differ materially from those described in these forward-looking statements due to certain factors, including changes in business, economic and competitive conditions, regulatory reforms, foreign exchange rate fluctuations, uncertainties in litigation or investigative proceedings, and the availability of financing. These and other risks and uncertainties are detailed in Fresenius Medical Care AG & Co. KGaA's reports filed with the U.S. Securities and Exchange Commission. Fresenius Medical Care AG & Co. KGaA does not undertake any responsibility to update the forward-looking statements in this release.
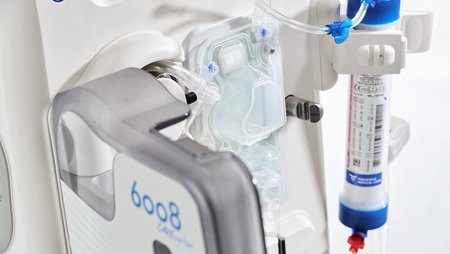
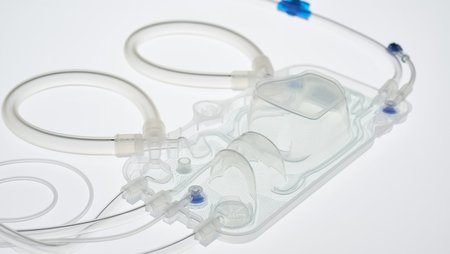
Fresenius Medical Care, the world’s largest provider of dialysis products and services, has won the Red Dot Award: Product Design for the company’s 6008 CAREset. This advanced all-in-one disposable with pre-connected bloodlines combines with the 6008 dialysis machine to form the 6008 CAREsystem. The 6008 CAREset was developed for use in all hemodialysis treatments, including Fresenius Medical Care’s advanced HighVolumeHDF dialysis therapy.
The Red Dot Award for outstanding international product design has been presented annually since 1955 by the Design Center North Rhine-Westphalia in Essen, Germany. More than 6,300 products from companies in 59 countries were entered into this year’s competition.
“The 6008 CAREsystem stands for reduced complexity, and minimizes the number of handling steps during dialysis,” said Dr. Olaf Schermeier, Fresenius Medical Care’s Chief Executive Officer for Global Research and Development. “The innovative design of the 6008 CAREset makes a key contribution to this. Because the bloodlines are pre-connected, the medical staff has more time to provide direct patient care. We are delighted about the recognition that this internationally renowned award will bring.”
Handling steps are reduced not only during dialysis, but during the set-up and disconnection phases before and after each treatment. The 6008 CAREset is simply inserted into the dialysis machine, eliminating the need to manually connect the bloodlines: This limits potential connection errors, which makes treatments safer. And in addition to being more cost efficient, the reduced amount of waste generated during a treatment makes the 6008 CAREsystem environmentally friendly.
Dialysis machines, bloodline systems and dialyzers – the latter often dubbed “artificial kidneys” because this is where the blood is actually cleaned – are the most important products for treating chronic kidney disease. During treatment, the dialysis machine pumps the patient’s blood through bloodlines, monitors its circulation through the dialyzer, and adds anti-coagulants. Treatments are generally carried out three times weekly, and take between three and six hours each.
About half of all dialysis machines and dialyzers sold worldwide are produced by Fresenius Medical Care.
More information about the Red Dot Award is available at https://en.red-dot.org.
Fresenius Medical Care is the world's largest provider of products and services for individuals with renal diseases of which around 3.2 million patients worldwide regularly undergo dialysis treatment. Through its network of 3,752 dialysis clinics, Fresenius Medical Care provides dialysis treatments for 320,960 patients around the globe. Fresenius Medical Care is also the leading provider of dialysis products such as dialysis machines or dialyzers. Along with the core business, the company focuses on expanding the range of related medical services in the field of Care Coordination. Fresenius Medical Care is listed on the Frankfurt Stock Exchange (FME) and on the New York Stock Exchange (FMS).
For more information visit the Company’s website at www.freseniusmedicalcare.com.
Disclaimer
This release contains forward-looking statements that are subject to various risks and uncertainties. Actual results could differ materially from those described in these forward-looking statements due to certain factors, including changes in business, economic and competitive conditions, regulatory reforms, foreign exchange rate fluctuations, uncertainties in litigation or investigative proceedings, and the availability of financing. These and other risks and uncertainties are detailed in Fresenius Medical Care AG & Co. KGaA's reports filed with the U.S. Securities and Exchange Commission. Fresenius Medical Care AG & Co. KGaA does not undertake any responsibility to update the forward-looking statements in this release.
- Targets 2017 achieved
- Strong revenue growth of 9% at constant currency
- Net income growth of 14% at constant currency
- Record dividend of EUR 1.06 for fiscal year 2017 proposed
- Strong net income growth for 2018 targeted
Key figures (IFRS) – fourth quarter and full year 2017
1 Net income attributable to shareholders of Fresenius Medical Care AG & Co. KGaA
cc = at constant currency
For a reconciliation of adjusted figures, please refer to the PDF.
“In 2017 we continued the success story of Fresenius Medical Care with another set of record results. We managed an unusual number of severe natural disasters, have delivered on our financial targets, and again we are able to propose the highest dividend in our Company’s history,” said Rice Powell, Chief Executive Officer of Fresenius Medical Care. “With the acquisition of the Cura Group in Australia and our planned acquisition of NxStage we are setting the course for future periods beyond our published 2020 outlook. We will continue improving our cost base with the implementation of the second phase of our Global Efficiency Program, GEP II. In 2018, we intend to continue the profitable growth track and further optimize our portfolio in the core dialysis as well as in the Care Coordination business.”
High net income growth for 2018 targeted
For 2018, Fresenius Medical Care expects revenue growth of around 8% at constant currency. The 2018 targets are based on 2017 revenue adjusted for the effect of the IFRS 15 implementation. Net income is expected to increase by 13 to 15% at constant currency including recurring benefits from the U.S. tax reform of EUR 140 to 160 million. The targets do not include effects from the NxStage acquisition. Fresenius Medical Care reconfirms the mid-term outlook for 2020, excluding the effect from IFRS 15 implementation and the recurring benefits from the U.S. tax reform in the years 2018 to 2020.
2 Numbers at constant currency
3 Reported revenue 2017 of EUR 17,784 million adjusted for effect from IFRS 15 implementation of EUR 486 million
4 Targets 2018: including recurring benefits from U.S. tax reform of EUR 140 to 160 million
21st consecutive dividend increase proposed
Based on the strong results for full year 2017, a dividend of EUR 1.06 per share, representing a dividend increase of 10%, will be proposed to the Annual General Meeting in May.
Strong underlying revenue and net income growth in 2017
Revenue in the fourth quarter 2017 – strongly impacted by headwinds from foreign exchange rates – came in at the level of the previous year’s quarter with EUR 4,429 million. At constant currency, revenue increased by 8% (+8% excluding the VA Agreement). Health Care Services revenue reached EUR 3,581 million and Health Care Products revenue came in at EUR 848 million. Both increased by 8% at constant currency.
Revenue for full year 2017 increased by 9% at constant currency to EUR 17,784 million (+9% excluding the VA Agreement). Health Care Services revenue increased by 10% at constant currency to EUR 14,532 million, mainly due to strong underlying organic growth and contributions from acquisitions. Health Care Products revenue increased by 7% at constant currency to EUR 3,252 million. This growth was primarily driven by higher sales of dialyzers, non-dialysis products in the acute business, machines and peritoneal dialysis products.
Corporate cost in the fourth quarter 2017 amounted to EUR 289 million. The strong increase compared to the fourth quarter 2016 (EUR 82 million) is mainly driven by the recognition of a charge of EUR 200 million based on ongoing discussions toward a settlement with the U.S. Securities and Exchange Commission and the U.S. Department of Justice that would avoid litigation over government demands under the Foreign Corrupt Practices Act related to certain identified conduct, including certain legal expenses and other related costs or asset impairments (“FCPA related charge”). In 2012, Fresenius Medical Care voluntarily advised the U.S. Department of Justice and the U.S. Securities Exchange Commission about its investigations into this conduct.
Operating income (EBIT) in the fourth quarter 2017 reached EUR 519 million. Adjusted EBIT increased by 6% at constant currency and reached EUR 726 million. For full year 2017, EBIT was EUR 2,362 million. On an adjusted basis EBIT increased by 5% at constant currency to EUR 2,493 million, mainly due to the strong business performance in North America and in Asia-Pacific.
Net interest expense in the fourth quarter 2017 was EUR 80 million, compared to EUR 90 million in the fourth quarter 2016. For full year 2017 net interest expense was EUR 354 million, a decrease of 3% year over year. The decrease was positively influenced by the replacement of interest bearing bonds, repaid in 2016 and 2017 by debt instruments at lower interest rates.
Income tax expense in the fourth quarter 2017 benefited from a book gain from the U.S. tax reform of EUR 236 million. Mainly for this reason, full year 2017 income tax expense decreased by 27% to EUR 454 million.
This decrease mainly resulted from the U.S. tax reform. Excluding (i) the impact from the VA Agreement, (ii) the effects associated with natural disaster costs, (iii) the FCPA related charge of EUR 200 million which was not tax effected and (iv) the U.S. tax reform, the 2017 effective tax rate increased to 31.0%, an increase of 50 basis points compared to the same period of 2016.
Net income5 for the fourth quarter of 2017 increased by 16% at constant currency to EUR 394 million. Adjusted for the impact from (i) the unfavorable effects of the VA Agreement (EUR 1 million), (ii) natural disaster costs in North America (EUR 3 million), (iii) FCPA related charge (EUR 200 million), as well as (iv) the benefit from the U.S. tax reform (EUR 236 million), net income was EUR 362 million (0%, +6% at constant currency). Based on approximately 306.9 million shares (weighted average number of shares outstanding), basic earnings per share (EPS) improved by 8%, to EUR 1.28. Adjusted for the effects described before, EPS was EUR 1.18 (0%, +6% at constant currency).
For 2017 net income5 increased by 14% at constant currency to EUR 1,280 million. Excluding the four effects described in the previous paragraph ((i) EUR +51 million, (ii) EUR -11 million, (iii) EUR -200 million, and (iv) EUR +236 million), net income increased to EUR 1,204 million (+5%, +7% at constant currency). Based on approximately 306.6 million shares, basic EPS increased from EUR 3.74 to EUR 4.17 (+12%). Excluding the effects described above, EPS increased to EUR 3.93 (+5%, +7% at constant currency).
5 Net income attributable to shareholders of Fresenius Medical Care AG & Co. KGaA
North America with strong growth supported by Care Coordination
In the fourth quarter 2017, the North America segment generated revenue of EUR 3,164 million (+8% at constant currency), strongly influenced by currency headwinds. Health Care Services revenue came in at EUR 2,950 million (+8% at constant currency), of which Care Coordination contributed EUR 715 million (+24% at constant currency) driven by significant organic revenue growth of 19%. Dialysis Care revenue reached EUR 2,235 million (+3% at constant currency). With $352, revenue per treatment in the United States was slightly down (-1%) due to lower revenue with commercial payors.
Cost per treatment increased by 3% to $276, largely driven by higher bad debt expenses, higher personnel expense and various other costs. The strong growth of the Health Care Products revenue (EUR 214 million, +9% at constant currency) was mainly driven by higher sales of machines, renal drugs and peritoneal dialysis products.
Operating income in North America in the fourth quarter reached EUR 608 million (+11% at constant currency). The operating income margin of 19.2% came in above last year’s strong fourth quarter margin of 18.4%. The improvement was triggered by an extraordinarily high contribution from Care Coordination which achieved an EBIT margin of 12.5%. The improvement was mainly driven by higher revenue including an acceleration of earnings from the Bundled Payment for Care Improvements (BPCI) initiative for previous reporting periods combined with increased volumes for hospital-related physician services, lower bad debt expense and the gain from the sale of Shiel Medical Laboratory. The Dialysis EBIT in North America reached EUR 519 million (-6% at constant currency), impacted by higher bad debt and personnel expenses, lower revenue with commercial payors, higher costs such as rent and insurance, the impact from natural disasters and higher costs for health care supplies.
For full year 2017, North America revenue increased by 9% at constant currency to EUR 12,879 million. Health Care Services revenue grew by 10% at constant currency to EUR 12,036 million, driven by higher Dialysis Care revenue (+5% at constant currency to EUR 9,227 million) and increased Care Coordination revenue (+28% at constant currency to EUR 2,809 million). Operating income increased in line with revenue growth to EUR 2,086 million (+10% at constant currency). As of the end of 2017, we had 197,356 patients being treated at the 2,393 clinics in North America. Dialysis treatments increased by 3%.
Solid Health Care Products and Health Care Services growth in EMEA
Revenue in the EMEA segment increased by 6% at constant currency to EUR 660 million in the fourth quarter 2017. Health Care Services revenue increased by 4% at constant currency to EUR 312 million. This was mainly the result of growth in same market treatments and contributions from acquisitions. Health Care Products revenue in EMEA increased by 7% at constant currency and reached EUR 348 million. The growth in Dialysis Products revenue was driven by higher sales of products for acute care, products for peritoneal dialysis and machines, partially offset by lower sales of dialyzers. Non-dialysis products increased due to higher sales of acute cardiopulmonary products. Operating income in the EMEA segment decreased by 7% at constant currency to EUR 110 million in the fourth quarter 2017. The operating income margin decreased year-over-year to 16.7% (Q4 2016: 19.0%) mainly due to further investments in Xenios and unfavorable foreign currency transaction effects.
For full year 2017, EMEA revenue increased by 6% at constant exchange rates to EUR 2,547 million and operating income decreased to EUR 444 million (-6% at constant currency). As of the end of 2017, we had 62,490 patients being treated at 746 clinics in EMEA. Dialysis treatments increased by 5%.
Growth in Asia-Pacific fuelled by acquisitions
Asia-Pacific revenue grew strongly by 12% at constant currency to EUR 418 million in the fourth quarter 2017. With EUR 191 million in Health Care Services revenue the region recorded growth of 17% at constant currency, mainly driven by the acquisition impact from Cura Group in Australia. The 7% constant currency growth in Health Care Products revenue to EUR 227 million was mainly supported by higher sales of dialyzers, bloodlines and products for peritoneal dialysis. Operating income reached EUR 76 million (-8% at constant currency). The operating income margin was 18.2% (Q4 2016: 21.8%). This was primarily driven by cost related to the build-up of dialysis services and peritoneal dialysis product business in China, the impact from foreign currency transaction effects and unfavorable mix effects related to acquisitions with lower margins.
For full year 2017, Asia-Pacific revenue grew by 13% at constant currency to EUR 1,623 million and operating income increased by 10% at constant currency to EUR 313 million. Operating income margin was stable on a high level of 19.3% (FY 2016: 19.6%). As of the end of 2017, we had 29,739 patients being treated at 381 clinics in Asia-Pacific. Dialysis treatments increased by 6%.
Improved contribution from Latin America
Latin America delivered revenue of EUR 185 million in the fourth quarter 2017, an increase of 16% at constant currency. Health Care Services revenue increased by 16% at constant currency to EUR 128 million and was driven by higher organic revenue per treatment. Health Care Products revenue increased by 15% at constant currency to EUR 57 million, mainly due to higher sales of machines, dialyzers and concentrates. Operating income came in at EUR 14 million (-12% at constant currency). The operating margin was 7.4% (Q4 2016: 9.7%), impacted by unfavorable foreign currency transaction effects, higher manufacturing costs primarily related to inflation, higher overhead costs and only partially offset by reimbursement rate increases that mitigate inflationary cost increases.
For full year 2017, Latin America revenue grew by 15% at constant currency to EUR 720 million and operating income increased by 3% at constant currency to EUR 58 million. Operating income margin was at 8.1% (FY 2016: 9.2%). As of the end of 2017, we had 31,375 patients being treated at 232 clinics in Latin America. Dialysis treatments increased by 2%.
Solid operating cash flow
In the fourth quarter 2017, the company generated net cash provided by operating activities of EUR 528 million, representing 11.9% of revenue (Q4 2016: EUR 772 million). The decrease was primarily attributable to a less favorable DSO (days sales outstanding) effect this year and higher income tax payments.
In full year 2017, the company generated net cash provided by operating activities of EUR 2,192 million, compared to EUR 1,932 million for full year 2016. This represents 12.3% of revenue, clearly reaching our 2017 target of more than 10%. The increase in net cash provided by operating activities was largely driven by the payment from the U.S. Departments of Veterans Affairs and Justice for reimbursement, the impact of the 2016 discretionary contribution of EUR 90 million to pension plan assets in the U.S. and the impact of other working capital items, partially offset by higher income tax payments. Free cash flow was also very strong at EUR 1,351 million, compared to EUR 1,017 million for full year 2016. DSO as of December 31, 2017 was 67 days, a decrease of 3 days compared to the previous year.
Global Efficiency Program phase II
Fresenius Medical Care has launched the second phase of its Global Efficiency Program (GEP II) in 2018. The program’s objectives are to identify and realize further efficiency potential and enhance the overall competitiveness of Fresenius Medical Care. Starting in 2018, GEP II targets to achieve sustained cost improvements of EUR 100 to 200 million per annum by 2020.
NxStage acquisition to foster home penetration
In August 2017, Fresenius Medical Care signed a merger agreement to acquire NxStage Medical, Inc., a U.S.-based medical technology and services company. The planned acquisition has a total transaction volume of approximately EUR 1.7 billion (USD 2.0 billion). On October 27, shareholders of NxStage approved the acquisition by Fresenius Medical Care. The completion of the acquisition is subject to regulatory approvals and other customary closing conditions. Closing is expected to occur in 2018.
Press Conference
Fresenius Medical Care will hold a press conference at its headquarters in Bad Homburg, Germany to discuss the results of the fourth quarter and full year 2017 on Tuesday, February 27, 2018, at 10 am CET. The press conference will be webcasted at the company's website. A replay will be available shortly after the conference.
Conference call
Fresenius Medical Care will hold a conference call to discuss the results of the fourth quarter and full year 2017 on Tuesday, February 27, at 3:30 p.m. CET / 9:30 a.m. EDT. The company invites investors to follow the live webcast of the call on the company’s website. A replay will be available shortly after the call.
Please refer to the PDF for a complete overview of the results for the fourth quarter and full year 2017.
Fresenius Medical Care is the world's largest provider of products and services for individuals with renal diseases of which around 3.2 million patients worldwide regularly undergo dialysis treatment. Through its network of 3,752 dialysis clinics, Fresenius Medical Care provides dialysis treatments for 320,960 patients around the globe. Fresenius Medical Care is also the leading provider of dialysis products such as dialysis machines or dialyzers. Along with the core business, the company focuses on expanding the range of related medical services in the field of Care Coordination. Fresenius Medical Care is listed on the Frankfurt Stock Exchange (FME) and on the New York Stock Exchange (FMS).
For more information visit the Company’s website at www.freseniusmedicalcare.com.
Disclaimers
This release contains forward-looking statements that are subject to various risks and uncertainties. Actual results could differ materially from those described in these forward-looking statements due to certain factors, including changes in business, economic and competitive conditions, regulatory reforms, foreign exchange rate fluctuations, uncertainties in litigation or investigative proceedings, and the availability of financing. These and other risks and uncertainties are detailed in Fresenius Medical Care AG & Co. KGaA's reports filed with the U.S. Securities and Exchange Commission. Fresenius Medical Care AG & Co. KGaA does not undertake any responsibility to update the forward-looking statements in this release.
Fresenius Medical Care, the world’s largest provider of dialysis products and services, expects material positive effects from the tax reform legislation signed into law in the United States today. The new law goes into effect on January 1, 2018.
In particular, the new legislation triggers the re-evaluation of deferred tax liabilities. This results in a one-time book gain of around 200 million Euros, to be reflected in 2017 Earnings After Tax.
On February 27, 2018 the company will release its 2017 business results and discuss the ongoing tax effect as part of its guidance for 2018.
Fresenius Medical Care is the world's largest provider of products and services for individuals with renal diseases of which around 3 million patients worldwide regularly undergo dialysis treatment. Through its network of 3,714 dialysis clinics, Fresenius Medical Care provides dialysis treatments for 317,792 patients around the globe. Fresenius Medical Care is also the leading provider of dialysis products such as dialysis machines or dialyzers. Along with the core business, the company focuses on expanding the range of related medical services in the field of Care Coordination. Fresenius Medical Care is listed on the Frankfurt Stock Exchange (FME) and on the New York Stock Exchange (FMS).
For more information visit the Company’s website at www.freseniusmedicalcare.com.
Disclaimer
This release contains forward-looking statements that are subject to various risks and uncertainties. Actual results could differ materially from those described in these forward-looking statements due to certain factors, including changes in business, economic and competitive conditions, regulatory reforms, foreign exchange rate fluctuations, uncertainties in litigation or investigative proceedings, and the availability of financing. These and other risks and uncertainties are detailed in Fresenius Medical Care AG & Co. KGaA's reports filed with the U.S. Securities and Exchange Commission. Fresenius Medical Care AG & Co. KGaA does not undertake any responsibility to update the forward-looking statements in this release.
Unicyte AG, a pioneering leader in human liver stem cells and nano-extracellular vesicles, announced today the appointment of Prof. Jonathan Knowles to its Scientific Advisory Board.
The board works closely with Unicyte’s management team to accelerate the company’s regenerative stem cell and extracellular vesicles programs for treating diabetes, non-alcoholic fatty liver disease, diabetic nephropathy and cancer. In addition, it provides scientific advice for the collaboration between Unicyte and Italy’s University of Turin in order to foster innovation and new research programs.
"We are excited to welcome Prof. Jonathan Knowles to our Scientific Advisory Board, as he has not only the scientific expertise but also the experience of developing highly innovative technologies in both pharma and biotech companies," said Florian Jehle, CEO of Unicyte and Vice President, Technology & Innovation Management within Research & Development at Fresenius Medical Care.
Jonathan Knowles is Chairman of the Board of Directors of Immunocore Ltd. and of the Access Committee of Genomics England. He is a Visiting Professor of Translational Medicine at the University of Oxford and formerly a distinguished Professor of Personalized Medicine at the University of Helsinki and at EPFL Lausanne. As Head of Research from 1997 to 2009 he had oversight of Research and Development for the Roche Group. He also served as a director on the boards of Genentech and of Chugai Pharmaceuticals, and from 1987 to 1997 was Director of the Glaxo Institute for Molecular Biology. Jonathan Knowles holds a 1st class honors degree in Biology from the University of East Anglia in England and a PhD in Molecular Genetics from the University of Edinburgh in Scotland.
Jonathan Knowles said: “I am excited to join Unicyte’s Scientific Advisory Board and to support the company as it is anticipating first partnerships for commercialization. Unicyte has a unique adult stem cell platform that overcomes the cost constraints of many alternative approaches and has significant market potential, especially in large indications such as diabetes. Also, I am very interested in the therapeutic potential of extracellular vesicles, and Unicyte has a lead in this area.”
Unicyte originated from the long-standing research collaboration between Fresenius Medical Care, the world's leading provider of products and services for individuals with renal diseases, and Prof. Giovanni Camussi, a leading expert in nano-extracellular vesicles and stem cells at the University of Turin. Now an independent affiliate of Fresenius Medical Care, Unicyte has a broad preclinical pipeline focusing on kidney and liver disorders, diabetes and oncology, and will work with partners when needed to advance these therapeutic programs.
Dr. Daniel Gau, Unicyte’s Head of Business Development, said: “Prof. Knowles will make an outstanding and valued addition to our Scientific Advisory Board, helping Unicyte to identify new areas of focus and potentially disruptive therapies for the benefit of our patients.”
Unicyte AG is a preclinical stage regenerative medicine company with a focus on kidney and liver disorders, diabetes and oncology. Unicyte evolved from a long-term research collaboration of Italy’s University of Turin and Fresenius Medical Care. Unicyte is headquartered in Oberdorf NW, Switzerland, and is an independent affiliate of Fresenius Medical Care, the world's largest provider of products and services for people with chronic kidney failure. For more information, visit Unicyte’s website at www.unicyte.ch.
Fresenius Medical Care is the world's largest provider of products and services for individuals with renal diseases of which around 3 million patients worldwide regularly undergo dialysis treatment. Through its network of 3,714 dialysis clinics, Fresenius Medical Care provides dialysis treatments for 317,792 patients around the globe. Fresenius Medical Care is also the leading provider of dialysis products such as dialysis machines or dialyzers. Along with the core business, the company focuses on expanding the range of related medical services in the field of Care Coordination. Fresenius Medical Care is listed on the Frankfurt Stock Exchange (FME) and on the New York Stock Exchange (FMS). For more information visit the Company’s website at www.freseniusmedicalcare.com.
The Molecular Biotechnology Center (MBC) at the University of Turin, active since September 2006, has the main objective to bring together investigators with different scientific backgrounds to facilitate an interdisciplinary approach to biomedical research. The Center is actively involved in biotechnological research in the field of biomedical sciences, with specific focus on the study of the molecular mechanisms at the basis of physiopathological processes that have a significant impact on human health, such as cardiovascular diseases, inflammation, cancer and stem cell biology. These research efforts are mainly based on the development of the most advanced molecular imaging technology, bioinformatic analysis and the generation of mouse and zebrafish models. For more information, visit www.mbc.unito.it/en.