mAbxience, a Fresenius Kabi majority-owned Group, today announced a strategic licensing agreement with Intas Pharmaceuticals Ltd for Etanercept biosimilar to target autoimmune diseases. Under the agreement, mAbxience will develop, manufacture, and supply the Etanercept biosimilar from its state-of-the-art, Good Manufacturing Practices (GMP)-approved facilities, and Intas will receive the rights to commercialize in more than 150 countries, including Europe and the United States.
This collaboration demonstrates mAbxience’s commitment to addressing the pressing need for innovative and affordable treatment options for autoimmune diseases.
mAbxience, a Fresenius Kabi majority-owned Group, today announced a strategic licensing agreement with Intas Pharmaceuticals Ltd for Etanercept biosimilar to target autoimmune diseases. Under the agreement, mAbxience will develop, manufacture, and supply the Etanercept biosimilar from its state-of-the-art, Good Manufacturing Practices (GMP)-approved facilities, and Intas will receive the rights to commercialize in more than 150 countries, including Europe and the United States.
This collaboration demonstrates mAbxience’s commitment to addressing the pressing need for innovative and affordable treatment options for autoimmune diseases.

An oncology day clinic from Quirónsalud doubles the number of patients, gives cancer patients precious time, and improves therapy at the same time – thanks to an integrated care model and digitalization. An outstanding example of how efficiency, quality and patient satisfaction can go hand in hand.
(Published: December 2023)
Providing care to more and more people while treating everyone individually with the right therapy. At the same time, working in a way that conserves resources for the nursing and medical teams, in the face of a shortage of specialists and in compliance with the highest medical standards. Especially in aging societies, hospitals today are facing ever greater challenges. This is also the case with the oncology day clinic at Madrid University Hospital Fundación Jiménez Díaz.
Significant improvement thanks to the new care model for oncology day patients
For a few years now, however, it has been demonstrating how to square this circle with a new care model for oncology day patients. The Jiménez Díaz is a 651-bed teaching hospital that belongs to the network of the private hospital chain Quirónsalud in Spain, which in turn is part of the Helios hospital group.
Operations at the day clinic in did not always run as smoothly as they do today. Just a few years ago, it was common for cancer patients to visit the various treatment stations they needed to go to independently and one after the other. Each department managed the appointments independently. As a result, the average treatment time for day patients often lasted ten hours spread over three days. This is common practice in most hospitals.
The medical teams of the individual wards were also less coordinated among themselves, and moreover burdened with administrative tasks. This was because the administrative burden for the more than 4,000 day patients per year was high. The same was true for the costs per treatment. "Listening to patients and our own doctors and nurses, we felt that we had to change something to streamline the process, to make it more efficient and convenient for our patients”, said Dr. Cristina Caramés.
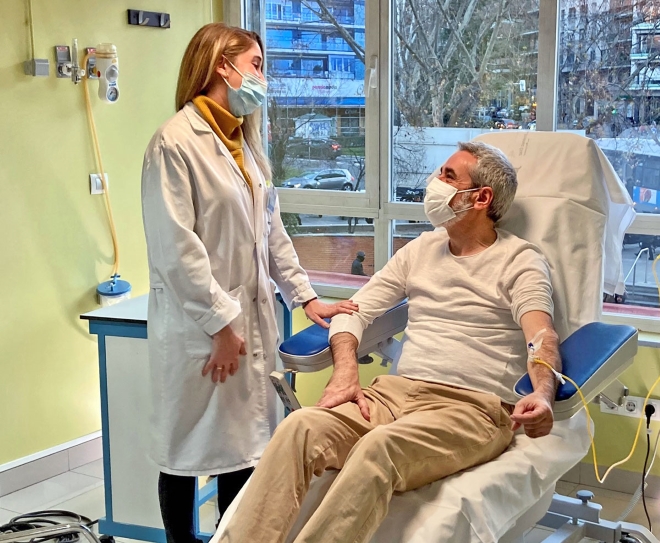
Situation today: less bureaucracy, better coordinated processes, less stress for everyone
Today, there is not only less bureaucracy and better interlocking processes. There's also less stress – for everyone involved. Thus, the therapies for the patients can also be much more effective, and their adherence has also increased. The corridors of the Quirónsalud day clinic are relaxed. Virtually no one has to wait long. Every patient and every nurse know what the next step is: after patient admission, first comes the examination in the laboratory, where patients receive their results within a few minutes, then the clinical evaluation where oncologists and specialized nurses give their recommendations for a therapy, then patients are potentially passing by the hospital pharmacy to receive medication. All in one visit and, what’s even more important, at the same place: the Oncology Day Clinic.
All appointments are planned digitally and timed so that one cog meshes with the other. As a result, total treatment time has been reduced by around 80 percent, from ten hours over three days to just two hours on a single day. The capacity freed up has made it possible to double the number of patients treated per day.
In 2018, the hospital management team and leading doctors had launched the HOPE initiative. The acronym HOPE means Hóspital Oncológico Personalizado in full. Its declared goal: to bundle as many steps of the cancer diagnosis and treatment offered as possible and to coordinate them more closely. At the same time, the treatment process was to be consolidated and streamlined in what is known as an integrated practice unit (IPU).
Day clinic guides patients through therapy as if on an almost perfectly planned journey
The whole thing was linked to a digitization initiative, which gave the transformation another major boost.
"We started by defining and describing each step of the treatment process," Dr. Caramés said. This allowed us to eliminate unproductive waiting time for patients. Building block by building block, the treatment process was streamlined further and further – without the people concerned missing anything. On the contrary. Today, the day clinic guides its patients through therapy as if on an almost perfectly planned journey, or "patient journey" in the technical jargon.
This profound change in treatment processes was backed up by a digitally supported central system that focuses on the systematic collection and processing of patient data. At any time, the system provides the information that the staff or the patients need, for example: When is the next appointment? What diagnostics are available? In this process, the day clinic records treatment data with an integrated electronic patient file, which is applied in-house.
Communication between the clinic and the patients is also predominantly digital today. The classic communication channels have been digitized and expanded. Today, medical staff and patients can communicate with each other at any time, even outside the hospital. The Quirónsalud "patient portal", an app that is available to all patients, serves as the central information and communication platform.
A virtual assistant gives individualized recommendations when patients have questions or need advice. It can also remind patients of appointments. A chatbot answers common questions, and in case of doubt, one can get quick online advice. "In emergencies, this service can be lifesaving," emphasizes Dr Caramés.
Thanks to the new communication options, we can also detect unwanted side effects at an early stage, in particular.

Number of hospital admissions reduced by more than 25 per cent
This option represents a very special benefit for patients and has contributed to the resounding success of the project. Above all, the systematization of data collection and the early detection of adverse effects have contributed to a reduction of more than 25 percent in the number of hospital admissions due to severe adverse effects or increased symptoms.
The improved service and streamlined procedures are well received by Madrid patients and their families: 98 percent of patients undergoing chemotherapy at the day clinic now use the integrated and personalized treatment. Compared to the past, they now have to visit the clinic much less often, thus gaining time and quality of life. The new system also offers enormous relief for the staff. "We are now always at the same level of knowledge, whether in nursing or in the medical service," explains Cristina Elez, a nursing specialist at the Jiménez Díaz. "This allows us to avoid duplicate work and time-consuming consultations." The medical staff thus has much more time for the really value-adding activities, but also to exchange a few words with the patients in between. And where the work gets off the ground so quickly, there is also an economic benefit: The day hospital was able to reduce the average cost per cancer treatment by about 24 percent.
As a result, the day hospital is already considered a model for outpatient oncology care. A best practice example of how day clinics can deliver on their promise despite increasing demands and growing patient numbers: to treat oncology patients according to the highest medical standards and with effective processes, while conserving the energies of the sick and the employees.
Amazing numbers
The HOPE project has improved many things at the Quirónsalud day clinic. For example:
- The number of oncology appointments decreased from 2018 to 2021 by more than 30,000 per year.
- In the same period, the clinic treated around four per cent more patients.
- The total treatment time was reduced from 10 hours over 3 days to only 2 hours on a single day
- The clinic can now treat up to 60 patients in a single day, twice as many as before
- While there used to be 16 appointments after an initial oncology consultation, there are now only 6 follow-up appointments. The so-called index of first-to-follow-up appointments, the number of appointments given after an initial appointment, was reduced by 63% from 16 to 6.
- The clinic team detects undesirable side effects earlier, so that those affected need to be hospitalized less often – instead of 58 percent of cases, it is currently 33 percent.
Related Links
QuirónsaludFresenius Helios will be linking digitalization even more closely with its core activities in inpatient and outpatient healthcare. The company will strengthen areas such as the digitalization of clinical processes and clinical decision making, for example through the responsible use of artificial intelligence.
Furthermore, a Digital Innovation Officer within the German Helios organization will explore innovative directions in digitalization. The implementation of digitalization processes and solutions will be the core task of the newly created function of the Head of Transformation Management at Helios. Also Quirónsalud in Spain is successfully driving forward the expansion of its leading digital processes.
In this context, Fresenius Helios will discontinue the activities of Curalie, which specializes in health apps, from the end of 2023. The Curalie subsidiaries meditec and ibs will be sold. Respective binding agreements have been signed. The business operations of the parent company Curalie GmbH and its other subsidiaries will be discontinued. The measures are in line with #FutureFresenius' intention to further focus business activities and reduce complexity.
Fresenius Helios will be linking digitalization even more closely with its core activities in inpatient and outpatient healthcare. The company will strengthen areas such as the digitalization of clinical processes and clinical decision making, for example through the responsible use of artificial intelligence.
Furthermore, a Digital Innovation Officer within the German Helios organization will explore innovative directions in digitalization. The implementation of digitalization processes and solutions will be the core task of the newly created function of the Head of Transformation Management at Helios. Also Quirónsalud in Spain is successfully driving forward the expansion of its leading digital processes.
In this context, Fresenius Helios will discontinue the activities of Curalie, which specializes in health apps, from the end of 2023. The Curalie subsidiaries meditec and ibs will be sold. Respective binding agreements have been signed. The business operations of the parent company Curalie GmbH and its other subsidiaries will be discontinued. The measures are in line with #FutureFresenius' intention to further focus business activities and reduce complexity.
Fresenius Kabi announced today it has signed a multiyear agreement under which the Mayo Clinic is expected to purchase 10,000 Ivenix large-volume infusion pumps for its hospitals and clinics in Minnesota, Arizona, and Florida. This is the largest contract for Ivenix pumps Fresenius Kabi has signed to date, testament of the stringent implementation of the company’s Vision 2026 strategy.
Fresenius Kabi announced today it has signed a multiyear agreement under which the Mayo Clinic is expected to purchase 10,000 Ivenix large-volume infusion pumps for its hospitals and clinics in Minnesota, Arizona, and Florida. This is the largest contract for Ivenix pumps Fresenius Kabi has signed to date, testament of the stringent implementation of the company’s Vision 2026 strategy.
- Fresenius makes use of the governmental compensation and reimbursement payments of up to €300 million (from the current perspective) provided for in the relief package for compensating additional costs caused by the increase in energy prices and implements the associated restrictions.
- Fresenius subsequently suspends dividend for fiscal year 2023; Fresenius Management Board members and management bodies of other companies covered by the statutory bans cannot be granted bonuses or other variable compensation components for fiscal year 2023.
- With the combination of the cash inflow of compensation and reimbursement payments and the dividend suspension, Fresenius reduces its leverage ratio (net debt/EBITDA) by around 20 to 25 basis points.
- Increasing the value of the company is clear priority on the way to #FutureFresenius.
Today, the Fresenius Management Board has decided to make use of the compensation and reimbursement payments for German hospitals in the amount of up to €300 million (from the current perspective) provided for by the "Energy Relief Package" (“Entlastungspaket Energiehilfen“) under the Hospital Financing Act (“Krankenhausfinanzierungsgesetz”) to cover increased energy costs. Today's decision of the Management Board is subject to the approval of the Supervisory Board of Fresenius Management SE, which is expected to decide on this matter on December 6, 2023. Fresenius' use of the compensation and reimbursement payments is subject to far-reaching conditions.
Fresenius therefore implements the related restrictions of the legislator. Fresenius Management Board will propose to the Annual General Meeting 2024 of Fresenius SE & Co. KGaA not to distribute a dividend for the fiscal year 2023. In addition, the Management Board members of Fresenius Management SE and management bodies of other companies covered by the statutory bans cannot be granted bonuses or other variable compensation components in the fiscal year 2023.
The decision is in line with the central objective of the #FutureFresenius strategy: the sustainable development and value enhancement of the company. The cash inflow of compensation and reimbursement payments as well as the dividend suspension will reduce the company's debt and consequently improve the leverage ratio by around 20 to 25 basis points. The reduction in debt will have a positive effect on net interest expense and ultimately on earnings per share. Furthermore, the compensation and reimbursement payments of up to €300 million (from the current perspective) will largely offset the additional costs of Helios Germany in 2023 caused directly or indirectly by the increase in energy prices.
"#FutureFresenius is our guideline for making and implementing strategically important decisions. Against this background, this is the right step for our company. We are strengthening our company’s intrinsic value. The lower level of debt affords us greater flexibility to make even better use of our market opportunities. We act with foresight and keep an eye on the sustainable development of the company and thus the future of patient care," said Fresenius CEO Michael Sen.
Fresenius is of the opinion that the statutory bans provided for in the "Energy Relief Package" („Entlastungspaket Energiehilfen“) are unconstitutional and that Fresenius' fundamental rights have been violated in view of the significant interference in the hospital financing system associated with this. Fresenius is therefore examining whether and in what form legal action should be taken.
In addition to operating performance improvement of the core business, the successfully progressing cost and efficiency program, the simplification of the Group structure through the deconsolidation of Fresenius Medical Care as well as the divestments of non-core businesses within the business segments, the compensation and reimbursement payments and the suspension of the dividend support the long-term strengthening of the Company.
Notwithstanding the legally required suspension of dividend payments for the fiscal year 2023, Fresenius maintains its dividend policy for the future. In line with its progressive dividend policy, Fresenius continues to aim to increase the dividend in line with growth in earnings per share (in constant currency, before special items), or at least maintain the dividend at the previous year's level.
This press release contains forward-looking statements that are subject to certain risks and uncertainties. Future results may differ substantially from those currently anticipated due to various risk factors and uncertainties, such as changes in the business, economic, and competitive situation, changes in legislation, results of clinical trials, exchange rate fluctuations, uncertainties regarding litigation or investigative proceedings, the availability of financing, and unforeseen effects of international conflicts. Fresenius assumes no responsibility to update the forward-looking statements contained in this press release.

