Quarterly Financial Report Q2 2024 (IFRS)
Quarterly Financial Report Q2 2024 (IFRS)
Share
Services
The Städel Museum provides unique access to over 700 years of art history. It offers space for a sensuous experience, and it encourages us to explore essential questions from our past, present, and future: The Städel Museum and its work are of utmost importance to society and to Frankfurt. For Fresenius, the partnership with Germany’s oldest museum foundation is part of its social responsibility – and a sign of its solidarity with the Rhine-Main region. After all, it was here that our company was founded and where both our corporate headquarters and many of our employees are based today.
“Fostering and funding the arts is an essential requirement for a diverse, open-minded, and pluralistic society,” says Fresenius CEO, Michael Sen. “Institutions like the Städel Museum ensure that everyone can access world-class works of art – a task that is also close to Fresenius’ heart. ‘Committed to Life’ – improving people’s lives – that is our claim. And both art and the Städel Museum make an important contribution to this.”
Studies show that art can have a positive impact on health. For example, the joy experienced when art is viewed can help to reduce stress. Above all, though, art fosters creativity and encourages people to reflect on social issues and different perspectives. The Städel Museum thus contributes to a diverse society with its work and helps to create the conditions fundamental to innovations that improve people’s lives.
Fresenius and the Städel Museum are thus also closely linked by the goals of innovation and progress. “I believe this attitude goes back to Johann Friedrich Städel and Else Kröner. Both bequeathed their fortunes to foundations that should advance progress in their respective disciplines. In the case of the Städel Museum, this means facilitating access to culture, also with the help of digital media. And in the case of the Else Kröner-Fresenius Foundation, this means furthering cutting-edge medical research,” continues Michael Sen. “I am therefore delighted to be working with such an outstanding cultural institution.”
Quarterly Financial Report Q2 2024 (IFRS)
Conference Call Q2/2024
1 Organic growth rate adjusted for accounting effects related to Argentina hyperinflation.
2 Before special items
3 Growth rate adjusted for Argentina hyperinflation
4 Excluding Fresenius Medical Care
5 At average exchange rates for both net debt and EBITDA; pro forma closed acquisitions/divestitures, including lease liabilities, including Fresenius Medical Care dividend
6 Constant currency
Conference call and Audio webcast
As part of the publication of the results for Q2/24, a conference call will be held on July 31, 2024 at 1:30 p.m. CEST (7:30 a.m. EDT). All investors are cordially invited to follow the conference call in a live audio webcast at www.fresenius.com/investors. Following the call, a replay will be available on our website.
Michael Sen, CEO of Fresenius: “Fresenius had an outstanding Q2 and first half in 2024. We delivered strong top-line growth, higher margins, and even more powerful bottom-line growth. Cash came in extremely strong, materially improving our financial profile. We are well ahead of our plans to deleverage and to take out costs. 2024 is an inflection year where we see how the work we’ve done continues to impact and improve the lives of patients and generate value for all stakeholders. Fresenius is 'Committed to Life' ".
Structural productivity improvements ahead of plan
The Group-wide cost and efficiency measures are progressing faster than planned. Including the first half of 2024, Fresenius has achieved structural cost savings totaling ~€336 million at EBIT level.
For the remainder of the year, Fresenius will continue its efforts to further increase its structural productivity. Some measures that were planned for 2025 will be brought forward to the current financial year. The company aims to achieve the target of annual sustainable cost savings of ~€400 million at EBIT level by year-end 2024. Originally, this was expected in 2025.
The structural cost savings continue to be driven by all business segments and the Corporate Center. Key elements include measures to reduce complexity, optimize supply chains and improve procurement processes.
Group sales and earnings development
Group revenue before special items increased by 6% (8% in constant currency) to €5,414 million (Q2/23: €5,113 million). Organic growth was 8%2 driven by an ongoing strong performance of Kabi and Helios. Currency translation had a negative effect of 2% on revenue growth.
Group EBITDA before special items increased by 14% (14% in constant currency) to €938 million (Q2/23: €822 million).
Group EBIT before special items increased by 16% (15% in constant currency) to €660 million (Q2/23: €571 million) mainly driven by the good earnings development at Kabi and Helios and the continued progress of the groupwide cost savings program. The EBIT margin before special items was 12.2% (Q2/23: 11.2%). Reported Group EBIT was €265 million (Q2/23: €187 million).
Group net interest before special items was -€108 million (Q2/23: -€99 million) mainly due to financing activities in a higher interest rate environment.
Group tax rate before special items was 26.1% (Q2/23: 25.2%).
1 Before special items
2 Organic growth rate adjusted for accounting effects related to Argentina hyperinflation
3 Net income attributable to shareholders of Fresenius SE & Co. KGaA
Constant currency growth rates adjusted for Argentina hyperinflation. Financial figures and growth rates adjusted for the divestment of the fertility services group Eugin and the hospital stake in Peru.
Net income1 from deconsolidated Fresenius Medical Care operations before special items increased by 21% (16% in constant currency) to €69 million (Q2/231: €57 million).
Group net income1 before special items increased by 16% (15% in constant currency) to €457 million (Q2/231: €393 million). The increase was driven by the operating strength.
Group net income1 before special items excluding Medical Care increased by 15% (15% in constant currency) to €388 million (Q2/231: €336 million).
Reported Group net income1 decreased to -€373 million (Q2/231: €80 million) due to effects from special items related to the Vamed exit and the discontinued operations at Vamed.
Earnings per share1 before special items increased by 16% (15% in constant currency) to €0.81 (Q2/231: €0.69). Reported earnings per share1 were -€0.66 (Q2/231: €0.15) effects from special items related to the Vamed exit and the discontinued operations at Vamed.
1 Net income attributable to shareholders of Fresenius SE & Co. KGaA
Constant currency growth rates adjusted for Argentina hyperinflation. Financial figures and growth rates adjusted for the divestment of the fertility services group Eugin and the hospital stake in Peru.
For a detailed overview of special items please see the reconciliation tables at Financial Results.
Group Cash flow development
Group operating cash flow (continuing operations) almost quintupled to €709 million (Q2/23: €148 million). This excellent development is mainly related to working capital efficiencies and the increased focus on cash generation as well as excellent operating performance in Spain at Fresenius Helios. Group operating cash flow margin was 13.1% (Q2/23: 2.9%). Free cash flow before acquisitions, dividends and lease liabilities (continuing operations) increased to €674 million (Q2/23: €40 million). Free cash flow after acquisitions, dividends and lease liabilities (continuing operations) improved to €655 million (Q2/23: -€556 million).
Fresenius Kabi’s operating cash flow increased to €259 million (Q2/23: €180 million) with a margin of 12.3% (Q2/23: 9.0%) mainly driven by an improved working capital management, in particular related to inventories and receivables.
Fresenius Helios’ operating cash flow increased to €604 million (Q2/23: €61 million) in particular due to the strong operating performance in Spain and catch-up effects following a weaker first quarter. The strong focus on cash generation and improved management of working capital is also paying off. The operating cash flow margin was 18.7% (Q2/23: 2.0%).
The cash conversion rate (CCR), which is defined as the ratio of adjusted free cash flow1 to EBIT before special items was 1.1 in Q2/24 (LTM) (Q1/24: 0.9 LTM). This positive development is due to the increased cash flow focus across the Group.
1 Cash flow before acquisitions and dividends; before interest, tax, and special items
Group leverage
Group debt decreased by 14% (-15% in constant currency) to €13,536 million (Dec. 31, 2023: € 15,830 million) mainly related to the repayment of debt and the €400 million reduction of the leasing liabilities related to the Vamed exit. Group net debt decreased by 6% (-7% in constant currency) to € 12,428 million (Dec. 31, 2023: € 13,268 million).
As of June 30, 2024, the net debt/EBITDA ratio was 3.43x1,2 (Dec. 31, 2023: 3.76x1,2) corresponding to a reduction of 33 bps compared to FY/23. This achievement is due to a combination of the improved operational performance as well as better EBITDA and Free cash flow. The legally required suspension of dividend payments and the Vamed exit further supported the positive development. Compared to Q2/23 (4.19x1,2) this is a 76 bps reduction.
Fresenius expects the net debt/EBITDA3 ratio to be within the self-imposed corridor of 3.0 to 3.5x by the end of 2024. Further improvement in the second half of 2024 is expected. This is expected to be driven by further reducing net debt and by the operational performance at the Operating Companies.
ROIC improved to 6.0% in Q2/24 (FY/23: 5.2%) mainly due to the EBIT improvement and the stringed capital allocation. With that, ROIC reached the lower end of the self-defined target range of 6% to 8%.
For 2024, Fresenius now expects ROIC to be around 6.0% (previous: 5.4% to 6.0%), (2023: 5.2%).
1 At average exchange rates for both net debt and EBITDA; pro forma closed acquisitions/divestitures, including lease liabilities, including Fresenius Medical Care dividend
2 Before special items
3 At expected average exchange rates for both net debt and EBITDA; pro forma closed acquisitions/divestitures; excluding further potential acquisitions/divestitures; before special items; including lease liabilities, including Fresenius Medical Care dividend
For a detailed overview of special items please see the reconciliation tables at Financial Results.
Operating Company Fresenius Kabi
Revenue increased by 5% (10% constant currency) to €2,101 million (Q2/23: €2,001 million). The reported revenue growth is mainly driven by negative currency translation effects related to the hyperinflation in Argentina. Organic growth was 11%1. This strong performance was driven by the Growth Vectors and helped by pricing effects in Argentina.
Revenue of the Growth Vectors (MedTech, Nutrition and Biopharma) increased by 8% (19% in constant currency) to €1,149 million (Q2/23: €1,062 million). Organic growth was outstanding at 19%1. In Nutrition, organic growth of 14%1 benefited from the good development in the US, driven by the ongoing roll-out of lipid emulsions. Whereas China continued to be impacted by indirect effects of the government’s countrywide anti-corruption campaign and tender headwinds. Biopharma showed excellent organic growth of 102%1 driven by licensing agreements at mAbxience and the successful product launch of Tyenne in Europe. In MedTech, organic growth was of 9%1 driven by a broad-based positive development across most regions and many products groups.
Revenue in the Pharma (IV Drugs & Fluids) business was flat (0% in constant currency; organic growth: 2%1) and amounted to €951 million (Q2/23: €952 million). Organic growth was mainly driven by the positive development across many regions, particularly Europe.
EBIT2 of Fresenius Kabi increased by 17% (17% in constant currency) to €334 million (Q2/23: €285 million) mainly due to the good revenue development, the EBIT break-even result of the Biopharma business, and ongoing progress of the cost saving initiatives. EBIT margin2 was 15.9% (Q2/23: 14.2%) and thus at the upper end of 2024 outlook.
EBIT2 of the Growth Vectors increased by 93% (constant currency: 47%) to €169 million (Q2/23: €88 million) due to the EBIT break-even result of the Biopharma business and the good revenue development. EBIT2 margin was 14.7% (Q2/23: 8.3%).
EBIT2 in the Pharma business decreased by 10% (constant currency: -11%) to €185 million (Q2/23: €206 million) primarily driven by additional costs due to the start of production at the main US plants in Wilson and Melrose Park. EBIT2 margin was 19.5% (Q2/23: 21.6%).
1 Organic growth rate adjusted for accounting effects related to Argentina hyperinflation.
2 Before special items
Constant currency growth rates adjusted for Argentina hyperinflation.
For a detailed overview of special items please see the reconciliation tables at Financial Results.
Operating Company Fresenius Helios
Revenue before special items increased by 7% (6% in constant currency) to €3,230 million (Q2/23: €3,020 million). Organic growth was 6%.
Revenue of Helios Germany increased by 3% (in constant currency: 3%) to €1,882 million (Q2/23: €1,823 million), mainly driven by favourable price effects and moderately increased activity levels. Organic growth was 3%.
Revenue of Helios Spain before special items increased by 13% (11% in constant currency) to €1,348 million (Q2/23: €1,198 million) driven by a positive calendar effect related to the Easter week and related higher activities, as well as positive price effects. Organic growth was 11%. The clinics in Latin America also showed a good performance.
EBIT1 of Fresenius Helios increased by 19% (18% in constant currency) to €357 million (Q2/23: €301 million) with an excellent EBIT margin1 of 11.1% (Q2/23: 10.0%) due to the strong operating performance in Spain.
EBIT1 of Helios Germany increased by 2% to €157 million (Q2/23: €154 million) with an EBIT margin1 of 8.3% (Q2/23: 8.4%) driven by the solid revenue development and helped by Government relief funding for higher energy costs.
EBIT1 of Helios Spain increased by 33% (32% in constant currency) to €201 million (Q2/23: €151 million) driven by the strong revenue growth based on the positive calendar effect related the Easter week as well as positive price effects. The EBIT margin1 reached 14.9% (Q2/23: 12.6%), clearly above the structural margin band ambition. On a more comparable half-year basis, the EBIT margin improved 30 bps to13.3% (H1/23: 13.0%).
Exit Fresenius Vamed
As of Q2 2024, Vamed is no longer a reporting segment of Fresenius. The company’s High-End-Services (HES) which offers services for Fresenius Helios and other hospitals, will be transferred to Fresenius and has already been included under Corporate / Other in the Group consolidated segment reporting.
The divestment of the rehabilitation business and the Vamed operations in Austria led to non-cash special items of €427 million at Group net income level.
Due to the exit from the project business, a total amount of high triple-digit million euros of special items are expected, which are spread over the next few years and mostly cash-effective. In H1/24, special items related to the gradual scale back of the international project business amounted to €425 million at Group EBIT level and to €343 million2 at Group net income level.
1 Before special items
2 According to 77% ownership share
Financial figures and growth rates adjusted for the divestment of the fertility services group Eugin and the hospital stake in Peru.
For a detailed overview of special items please see the reconciliation tables at Financial Results.
Group and segment outlook for 20241
Fresenius confirms its outlook for FY/24. Based on the excellent first half year, Fresenius is optimistic to get Group constant currency EBIT2,3 growth into the upper half of the 6% to 10% range. For 2024, Group organic revenue growth2,3 is expected to grow between 4% to 7%.
Fresenius Kabi expects organic revenue growth in a mid-to high-single-digit percentage range in 2024. The EBIT margin3 is expected to be in a range of 15% to 16% (structural margin band: 14% to 17%).
Fresenius Helios expects organic revenue3 to grow in mid-single digit percentage range in 2024. The EBIT margin3 is expected to be within 10% to 11% (structural margin band: 10% to 12%).
The Group outlook is given without Fresenius Vamed, i.e. exclusively for the Operating Companies Fresenius Kabi and Fresenius Helios.
1 For the prior-year basis please see table “Basis for Guidance for 2024”
2 2023 base: €2,266 million
3 Before special items
4 2023 base: €20,307 million
Basis for Guidance for 2024
1 Before special items
If no timeframe is specified, information refers to Q2/2024.
An overview of the results for Q2/2024 - before and after special items – is available on our website.
Consolidated results for Q2/24 as well as for Q2/23 include special items. These concern: divestment of the fertility services group Eugin and the hospital stake in Peru, Vamed exit, expenses associated with the Fresenius cost and efficiency program, transaction costs for mAbxience and Ivenix, costs in relation to the change of legal form of Fresenius Medical Care, legacy portfolio adjustments, IT transformation, transformation/exit Vamed, discontinued operations Vamed, special items at Fresenius Medical Care, and impact of PPA due to the application of the equity method to the Fresenius Medical Care investment. The special items shown within the reconciliation tables are reported in the Corporate/Other segment.
Note on the deconsolidation of Fresenius Medical Care
Following the deconsolidation of Fresenius Medical Care, Group financial figures are presented in accordance with IAS 28 (at equity method) since December 1, 2023. The proportionate share of 32% of Fresenius Medical Care is presented as a separate line in Fresenius Group’s P&L and balance sheet. Dividends received from Fresenius Medical Care are reported as a separate line as part of the cash flow statement. Moreover, IAS 28 requires a full purchase price allocation (PPA). The accounting for the PPA is treated as special item. For reasons of simplification and comparability, Fresenius presents net income with and without Fresenius Medical Care`s equity result.
Note on the portfolio optimization at Fresenius Helios
As part of the portfolio optimization, the sale of the fertility services group Eugin was completed on January 31, 2024. The divestment of the majority stake in the hospital Clínica Ricardo Palma hospital in Lima, Peru, was completed on April 23, 2024. Therefore, results of Fresenius Helios and accordingly of the Fresenius Group for Q2/24 and Q2/23 are adjusted.
Note on the growth rates Fresenius Kabi
Growth rates in constant currency of Fresenius Kabi are adjusted. Adjustments relate to the hyperinflation in Argentina. Accordingly, in constant currency growth rates of the Fresenius Group are also adjusted.
Note on the Vamed exit
Due to the application of IFRS 5, the prior year and prior quarter figures of the current year have been adjusted in the consolidated statement of income and the consolidated statement of cash flows. Vamed’s High-End-Services (HES) which offers services for Fresenius Helios and other hospitals, will be transferred to Fresenius and is included under Corporate / Other in the Group consolidated segment reporting. Details on the financial and accounting implications of the Vamed exit and the portfolio adjustments at Fresenius Helios are available on our website.
Information on the performance indicators are available on our website at
https://www.fresenius.com/alternative-performance-measures.
This release contains forward-looking statements that are subject to various risks and uncertainties. Future results could differ materially from those described in these forward-looking statements due to certain factors, e.g. changes in business, economic and competitive conditions, regulatory reforms, results of clinical trials, foreign exchange rate fluctuations, uncertainties in litigation or investigative proceedings, the availability of financing and unforeseen impacts of international conflicts. Fresenius does not undertake any responsibility to update the forward-looking statements in this release.
1 Organic growth rate adjusted for accounting effects related to Argentina hyperinflation
2 Before special items
3 Growth rate adjusted for Argentina hyperinflation
4 Excluding Fresenius Medical Care
5 At average exchange rates for both net debt and EBITDA; pro forma closed acquisitions/divestitures, including lease liabilities, including Fresenius Medical Care dividend
6 Constant currency
If no timeframe is specified, information refers to Q2/2024.
Michael Sen, CEO of Fresenius: “Fresenius had an outstanding Q2 and first half in 2024. We delivered strong top-line growth, higher margins, and even more powerful bottom-line growth. Cash came in extremely strong, materially improving our financial profile. We are well ahead of our plans to deleverage and to take out costs. 2024 is an inflection year where we see how the work we’ve done continues to impact and improve the lives of patients and generate value for all stakeholders. Fresenius is ‘Committed to Life‘.“
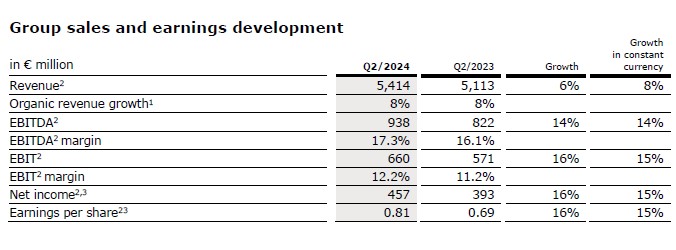
Group revenue before special items increased by 6% (8% in constant currency) to €5,414 million (Q2/23: €5,113 million). Organic growth was 8%1 driven by an ongoing strong performance of Kabi and Helios. Currency translation had a negative effect of 2% on revenue growth.
Group EBITDA before special items increased by 14% (14% in constant currency) to €938 million (Q2/23: €822 million).
1 Organic growth rate adjusted for the accounting effects related to Argentina hyperinflation
2 Before special items
3 Net income attributable to shareholders of Fresenius SE & Co. KGaA
Constant currency growth rates adjusted for Argentina hyperinflation. Financial figures and growth rates adjusted for the divestment of the fertility services group Eugin and the hospital stake in Peru
Group EBIT before special items increased by 16% (15% in constant currency) to €660 million (Q2/23: €571 million) mainly driven by the good earnings development at Kabi and Helios and the continued progress of the groupwide cost cost and efficiency program. The EBIT margin before special items was 12.2% (Q2/23: 11.2%). Reported Group EBIT was €265 million (Q2/23: €187 million).
Group net interest before special items was -€108 million (Q2/23: -€99 million) mainly due to financing activities in a higher interest rate environment.
Group tax rate before special items was 26.1% (Q2/23: 25.2%).
Net income1 from deconsolidated Fresenius Medical Care operations before special items increased by 21% (16% in constant currency) to €69 million (Q2/231: €57 million).
Group net income1 before special items increased by 16% (15% in constant currency) to €457 million (Q2/231: €393 million). The increase was driven by the operating strength.
Group net income1 before special items excluding Fresenius Medical Care increased by 15% (15% in constant currency) to €388 million (Q2/231: €336 million).
Reported Group net income1 decreased to -€373 million (Q2/231: €80 million) due to effects from special items related to the Vamed exit and the discontinued operations at Vamed.
Earnings per share2 before special items increased by 16% (15% in constant currency) to €0.81 (Q2/231: €0.69). Reported earnings per share1 were -€0.66 (Q2/231: €0.15) effects from special items related to the Vamed exit and the discontinued operations at Vamed.
1 Net income attributable to shareholders of Fresenius SE & Co. KGaA
Constant currency growth rates adjusted for Argentina hyperinflation. Financial figures and growth rates adjusted for the divestment of the fertility services group Eugin and the hospital stake in Peru
For a detailed overview of special items please see the reconciliation tables at
Financial Results | FSE (fresenius.com)
2 Net income attributable to shareholders of Fresenius SE & Co. KGaA
Group cash flow development
Group operating cash flow (continuing operations) almost quintupled to €709 million (Q2/23: €148 million). This excellent development is mainly related to working capital efficiencies and the increased focus on cash generation as well as excellent operating performance in Spain at Fresenius Helios. Group operating cash flow margin was 13.1% (Q2/23: 2.9%). Free cash flow before acquisitions, dividends and lease liabilities (continuing operations) increased to €674 million (Q2/23: €40 million). Free cash flow after acquisitions, dividends and lease liabilities (continuing operations) improved to €655 million (Q2/23: -€556 million).
Fresenius Kabi’s operating cash flow increased to €259 million (Q2/23: €180 million) with a margin of 12.3% (Q2/23: 9.0%) mainly driven by an improved working capital management, in particular related to inventories and receivables.
Fresenius Helios’ operating cash flow increased to €604 million (Q2/23: €61 million) in particular due to the strong operating performance in Spain and catch-up effects following a weaker first quarter. The strong focus on cash generation and improved management of working capital is also paying off. The operating cash flow margin was 18.7% (Q2/23: 2.0%).
Group leverage
Group debt decreased by 14% (-15% in constant currency) to €13,536 million
(Dec. 31, 2023: € 15,830 million) mainly related to the repayment of debt and the €400 million reduction of the leasing liabilities related to the Vamed exit. Group net debt decreased by 6% (-7% in constant currency) to € 12,428 million (Dec. 31, 2023: € 13,268 million).
As of June 30, 2024, the net debt/EBITDA ratio was 3.43x1,2 (Dec. 31, 2023: 3.76x1,2) corresponding to a reduction of 33 bps compared to FY/23. This achievement is due to a combination of the improved operating performance as well as better EBITDA and free cash flow. The legally required suspension of dividend payments and the Vamed exit further supported the positive development. Compared to Q2/23 (4.19x1,2) this is a 76 bps reduction.
Fresenius expects the net debt/EBITDA3 ratio to be within the self-imposed corridor of 3.0 to 3.5x by the end of 2024. Further improvement in the second half of 2024 is expected. This is expected to be driven by further reducing net debt and by the operating performance at the Operating Companies.
Structural productivity improvements ahead of plan
The Group-wide cost and efficiency measures are progressing faster than planned. Including the first half of 2024, Fresenius has achieved structural cost savings totaling ~€336 million at EBIT level.
For the remainder of the year, Fresenius will continue its efforts to further increase its structural productivity. Some measures that were planned for 2025 will be brought forward to the current financial year. The company aims to achieve the target of annual sustainable cost savings of ~€400 million at EBIT level by year-end 2024. Originally, this was expected in 2025.
The structural cost savings continue to be driven by all business segments and the Corporate Center. Key elements include measures to reduce complexity, optimize supply chains, and improve procurement processes.
1 At average exchange rates for both net debt and EBITDA; pro forma closed acquisitions/divestitures, including lease liabilities, including Fresenius Medical Care dividend
2 Before special items
3 At expected average exchange rates for both net debt and EBITDA; pro forma closed acquisitions/divestitures; excluding further potential acquisitions/divestitures; before special items; including lease liabilities, including Fresenius Medical Care dividend
For a detailed overview of special items please see the reconciliation tables at
Financial Results | FSE (fresenius.com)
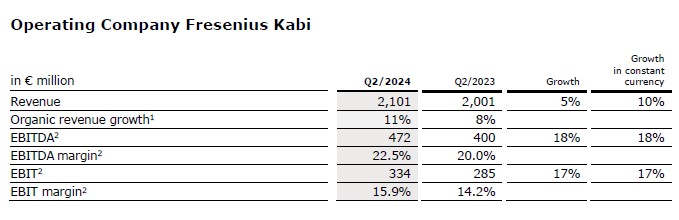 Revenue of Kabi increased by 5% (10% constant currency) to €2,101 million (Q2/23: €2,001 million). The reported revenue growth is mainly driven by negative currency translation effects related to the hyperinflation in Argentina. Organic growth was 11%1. This strong performance was driven by the Growth Vectors and helped by pricing effects in Argentina.
Revenue of Kabi increased by 5% (10% constant currency) to €2,101 million (Q2/23: €2,001 million). The reported revenue growth is mainly driven by negative currency translation effects related to the hyperinflation in Argentina. Organic growth was 11%1. This strong performance was driven by the Growth Vectors and helped by pricing effects in Argentina.
Revenue of the Growth Vectors (MedTech, Nutrition and Biopharma) increased by 8% (19% in constant currency) to €1,149 million (Q2/23: €1,062 million). Organic growth was outstanding at 19%1. In Nutrition, organic growth of 14%1 benefited from the good development in the US, driven by the ongoing roll-out of lipid emulsions. Whereas China continued to be impacted by indirect effects of the government’s countrywide anti-corruption campaign and tender headwinds. Biopharma showed excellent organic growth of 102%1 driven by licensing agreements at mAbxience and the successful product launch of Tyenne in Europe. In MedTech organic growth was of 9%1 driven by a broad-based positive development across most regions and many products groups.
Revenue in the Pharma (IV Drugs & Fluids) business was flat (0% in constant currency; organic growth: 2%1) and amounted to €951 million (Q2/23: €952 million). Organic growth was mainly driven by the positive development across many regions, particularly Europe.
EBIT2 of Fresenius Kabi increased by 17% (17% in constant currency) to €334 million (Q2/23: €285 million) mainly due to the good revenue development, the EBIT break-even result of the Biopharma business, and ongoing progress of the cost saving initiatives.
1 Organic growth rate adjusted for accounting effects related to Argentina hyperinflation.
2 Before special items
Constant currency growth rates adjusted for Argentina hyperinflation.
For a detailed overview of special items please see the reconciliation tables at
Financial Results | FSE (fresenius.com)
EBIT margin1 was 15.9% (Q2/23: 14.2%) and thus at the upper end of 2024 outlook.
EBIT1 of the Growth Vectors increased by 93% (constant currency: 47%) to €169 million (Q2/23: €88 million) due to the EBIT break-even result of the Biopharma business and the good revenue development. EBIT1 margin was 14.7% (Q2/23: 8.3%).
EBIT1 in the Pharma business decreased 10% (constant currency: -11%) to €185 million (Q2/23: €206 million) primarily driven by additional costs due to the start of production at the main US plants in Wilson and Melrose Park. EBIT1 margin was 19.5% (Q2/23: 21.6%).
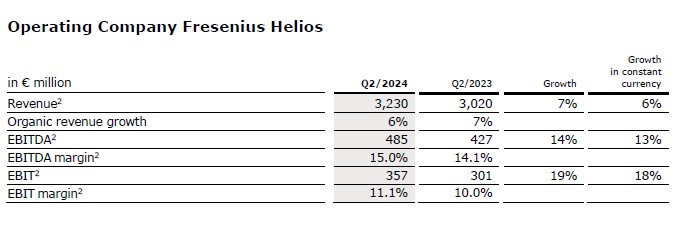
Revenue before special items increased by 7% (6% in constant currency) to €3,230 million (Q2/23: €3,020 million). Organic growth was 6%.
Revenue of Helios Germany increased by 3% (in constant currency: 3%) to €1,882 million (Q2/23: €1,823 million), mainly driven by favourable price effects and moderately increased activity levels. Organic growth was 3%.
1 Before special items
Constant currency growth rates adjusted for Argentina hyperinflation
For a detailed overview of special items please see the reconciliation tables at
Financial Results | FSE (fresenius.com)
2 Before special items
Financial figures and growth rates adjusted for the divestment of the fertility services group Eugin and the hospital stake in Peru
For a detailed overview of special items please see the reconciliation tables at
Financial Results | FSE (fresenius.com)
Revenue of Helios Spain before special items increased by 13% (11% in constant currency) to €1,348 million (Q2/23: €1,198 million) driven by a positive calendar effect related to the Holy week and related higher activities, as well as positive price effects. Organic growth was 11%. The clinics in Latin America also showed a good performance.
EBIT1 of Fresenius Helios increased by 19% (18% in constant currency) to €357 million (Q2/23: €301 million) with an excellent EBIT margin1 of 11.1% (Q2/23: 10.0%) due to the strong operating performance in Spain.
EBIT1 of Helios Germany increased by 2% to €157 million (Q2/23: €154 million) with an EBIT margin1 of 8.3% (Q2/23: 8.4%) driven by the solid revenue development and helped by Government relief funding for higher energy costs.
EBIT1 of Helios Spain increased by 33% (32% in constant currency) to €201 million (Q2/23: €151 million) driven by the strong revenue growth based on the positive calendar effect related the Holy week as well as positive price effects. The EBIT margin1 reached 14.9% (Q2/23: 12.6%), clearly above the structural margin band ambition. On a more comparable half-year basis, the EBIT margin improved 30 bps to 13.3% (H1/23: 13.0%).
Fresenius Vamed Exit
As of Q2 2024, Vamed is no longer a reporting segment of Fresenius. Vamed’s High-End-Services (HES) which offers services for Fresenius Helios and other hospitals, will be transferred to Fresenius and has already been included under Corporate / Other in the Group consolidated segment reporting.
The divestment of the rehabilitation business and the Vamed operations in Austria led to non-cash special items of €427 million at Group net income level.
1 Before special items
Financial figures and growth rates adjusted for the divestment of the fertility services group Eugin and the hospital stake in Peru
For a detailed overview of special items please see the reconciliation tables at
Financial Results | FSE (fresenius.com)
Due to the exit from the project business, a total amount of high triple-digit million euros of special items are expected, which are spread over the next few years and mostly cash-effective. In H1/24, special items related to the gradual scale back of the international project business amounted to €425 million at Group EBIT level and to €343 million at Group net income level.
Group and segment outlook for 2024
Fresenius confirms its outlook for FY/24. Based on the excellent first half year, Fresenius is optimistic to get Group constant currency EBIT2,3 growth into the upper half of the 6% to 10% range. For 2024, Group organic revenue growth3,4 is expected to grow between 4% to 7%.
Fresenius Kabi expects organic revenue growth in a mid-to high-single-digit percentage range in 2024. The EBIT margin3 is expected to be in a range of 15% to 16% (structural margin band: 14% to 17%).
Fresenius Helios expects organic revenue3 to grow in mid-single digit percentage range in 2024. The EBIT margin3 is expected to be within 10% to 11% (structural margin band: 10% to 12%).
The Group outlook is given without Fresenius Vamed, i.e. exclusively for the Operating Companies Fresenius Kabi and Fresenius Helios.
1 According to 77% ownership share
2 2023 base: €2,266 million
3 Before special items
4 2023 base: €20,307 million
Consolidated results for Q2/24 as well as for Q2/24 include special items. These concern: divestment of the fertility services group Eugin and the hospital stake in Peru, Vamed exit, expenses associated with the Fresenius cost and efficiency program, transaction costs for mAbxience and Ivenix, costs in relation to the change of legal form of Fresenius Medical Care, legacy portfolio adjustments, IT transformation, transformation/exit Vamed, discontinued operations Vamed, special items at Fresenius Medical Care, and impact of PPA due to the application of the equity method to the Fresenius Medical Care investment.
An overview of the results for Q2/24 - before and after special items – is available on our website. Information on the performance indicators are available on our website at https://www.fresenius.com/alternative-performance-measures.
Note on the deconsolidation of Fresenius Medical Care
Following the deconsolidation of Fresenius Medical Care, Group financial figures are presented in accordance with IAS 28 (at equity method) since December 1, 2023. The proportionate share of 32% of Fresenius Medical Care is presented as a separate line in Fresenius Group’s P&L and balance sheet. Dividends received from Fresenius Medical Care are reported as a separate line as part of the cash flow statement. Moreover, IAS 28 requires a full purchase price allocation (PPA). The accounting for the PPA is treated as special item. For reasons of simplification and comparability, Fresenius presents net income with and without Fresenius Medical Care`s equity result.
Note on the portfolio optimization at Fresenius Helios
As part of the portfolio optimization, the sale of the fertility services group Eugin was completed on January 31, 2024. The divestment of the majority stake in the hospital Clínica Ricardo Palma hospital in Lima, Peru, was completed on April 23, 2024. Therefore, results of Fresenius Helios and accordingly of the Fresenius Group for Q2/24 and Q2/23 are adjusted.
Note on the growth rates Fresenius Kabi
Growth rates in constant currency of Fresenius Kabi are adjusted. Adjustments relate to the hyperinflation in Argentina. Accordingly, in constant currency growth rates of the Fresenius Group are also adjusted.
Note on the Vamed exit
Due to the application of IFRS 5, the prior year and prior quarter figures of the current year have been adjusted in the consolidated statement of income and the consolidated statement of cash flows. Vamed’s High-End-Services (HES) which offers services for Fresenius Helios and other hospitals, will be transferred to Fresenius and is included under Corporate / Other in the Group consolidated segment reporting. Details on the financial and accounting implications of the Vamed exit and the portfolio adjustments at Fresenius Helios are available on our website.
Conference call and Audio webcast
As part of the publication of the results for Q2/24, a conference call will be held on July 31, 2024 at 1:30 p.m. CEST (7:30 a.m. EDT). All investors are cordially invited to follow the conference call in a live audio webcast at https://www.fresenius.com/investors. Following the call, a replay will be available on our website.
Fresenius SE & Co. KGaA (Frankfurt/Xetra: FRE) is a global healthcare company headquartered in Bad Homburg v. d. Höhe, Germany. In the 2023 fiscal year, Fresenius generated €22.3 billion in annual revenue with its more than 190,000 employees. Fresenius offers solutions to the social challenges posed by a growing and ageing population and the resulting need for affordable, high-quality healthcare. The Fresenius Group comprises the operating companies Fresenius Kabi and Fresenius Helios as well as the investment company Fresenius Medical Care. With 140 hospitals and countless outpatient facilities, Fresenius Helios is the leading private hospital operator in Germany and Spain, treating around 26 million patients every year. Fresenius Kabi’s product portfolio includes a range of highly complex biopharmaceuticals, clinical nutrition, medical technology, and generic intravenous drugs. Fresenius was established in 1912 by the Frankfurt pharmacist Dr. Eduard Fresenius. After his death, Else Kröner took over management of the company in 1952. She laid the foundations for a global enterprise that today pursues the goal of improving people’s health. The largest shareholder is the non-profit Else Kröner-Fresenius Foundation, which is dedicated to advancing medical research and supporting humanitarian projects.
September 24, 2024
Munich, Germany