Fresenius Medical Care, the world's leading provider of products and services for individuals with renal diseases, resolved a legal dispute with the U.S. government, for accounts receivable in legal dispute, and entered into a final and legally binding settlement agreement today, whereby the company will receive a payment from the U.S. government.
Fresenius Medical Care had filed a complaint against the United States in 2019. This complaint sought to recover monies owed to the company by the U. S. Department of Defense under the Tricare program, for services on or before January 11, 2023.
Tricare provides reimbursement for dialysis treatments and other medical care provided to members of the military services, their dependents and retirees. The litigation challenged unpublished administrative actions by Tricare administrators to reduce the rate of compensation paid for dialysis treatments provided to Tricare beneficiaries based on a recasting of invoicing codes. Tricare administrators had acknowledged the unpublished administrative action and declined to change or abandon it.
The now executed settlement agreement resolves the dispute underlying the complaint and concludes the litigation.
As a consequence of the settlement agreement, both revenue and operating income will be positively impacted. In previous reporting periods, the negative impact related to this matter had not been treated as special item due to its operational nature. Fresenius Medical Care therefore expects a net positive impact on operating income (guidance basis)1 of approx. EUR 175 million in the 4th quarter 2023.
The company had previously expected in fiscal year 2023 operating income (guidance basis)1 to grow by a low-single-digit percentage rate, compared to previous year (2022 basis: EUR 1,540 million).
As a consequence of the settlement agreement, Fresenius Medical Care today raises its earnings outlook. The company now expects operating income (guidance basis)1 to grow by 12 to 14 percent in fiscal year 2023, compared to previous year. All other elements of the 2023 outlook, as published, remain unchanged.
In line with its disciplined financial policy, Fresenius Medical Care intends to use the agreed settlement payment to reduce its net financial debt and therefore deleverage the balance sheet.
1 Operating income, as presented in the outlook, is on a constant currency basis and excluding special items. Special items will be provided as separate KPI (“Operating income excluding special items”) to capture effects that are unusual in nature and have not been foreseeable or not foreseeable in size or impact at the time of giving guidance. These items are excluded to ensure comparability of the figures presented with the Company’s financial targets which have been defined excluding special items.
As described in FME’s public reports for FY 2022, special items included costs related to the FME25 program, the impact of the war in Ukraine, the impact of hyperinflation in Turkiye, the Humacyte investment remeasurement and the net gain related to InterWell Health. Additionally, the FY 2022 basis for the 2023 outlook was adjusted for U.S. Provider Relief Funding. For FY 2023, special items include costs related to the FME25 program, the Humacyte investment remeasurement, the costs associated with the legal form conversion and effects from legacy portfolio optimization.
This release contains forward-looking statements that are subject to various risks and uncertainties. Actual results could differ materially from those described in these forward-looking statements due to various factors, including, but not limited to, changes in business, economic and competitive conditions, legal changes, regulatory approvals, impacts related to the COVID-19 pandemic results of clinical studies, foreign exchange rate fluctuations, uncertainties in litigation or investigative proceedings, and the availability of financing. These and other risks and uncertainties are detailed in Fresenius Medical Care AG & Co. KGaA's reports filed with the U.S. Securities and Exchange Commission. Fresenius Medical Care AG & Co. KGaA does not undertake any responsibility to update the forward-looking statements in this release.
- Continued solid organic growth in Care Enablement and Care Delivery including sequentially stable same market treatment growth in the U.S.
- Successful execution on turnaround plan driving productivity improvements in Care Delivery and pricing in Care Enablement
- FME25 transformation savings fully on track
- Continued execution on portfolio optimization strategy
- FY 2023 earnings outlook raised
Helen Giza, Chief Executive Officer of Fresenius Medical Care, said: “Our unwavering focus on executing against our strategic plan and the successful implementation of turnaround measures to date, continue to translate into improved operating performance. Notably in the third quarter, we continued to unlock sustainable savings with our FME25 program, further improved our labor productivity and continued to execute on our portfolio optimization plan. Given our improving performance for the first nine months of the year and solid expectations for the remainder of the year, we confidently upgrade our full year earnings outlook.”

Successful execution against the strategic plan
Fresenius Medical Care has continuously advanced its structural change. After implementing the new operating model along with the corresponding new financial reporting, the simplification of the governance structure through a legal form conversion remains on track to be completed by 1 December 2023.
Fresenius Medical Care continues to successfully execute on its operational efficiency and turnaround plans. In the third quarter, the FME25 transformation program delivered EUR 97 million of savings, resulting in EUR 232 million for the first nine months of the year. The Company is fully on track to achieve sustainable savings of EUR 250 to 300 million by year end 2023 and EUR 650 million by year end 2025.
Moreover, Fresenius Medical Care is executing its portfolio optimization plan to exit non-core and dilutive assets. In the third quarter, the Company entered into an agreement to sell National Cardiovascular Partners (NCP) with 21 facilities providing outpatient cardiac catheterization and vascular laboratory services, which are included in the U.S. Care Delivery business, in connection with its Legacy Portfolio Optimization program.
1 For FY 2022, special items included costs related to the FME25 program, the impact of the war in Ukraine, the impact of hyperinflation in Turkiye, the Humacyte investment remeasurement and the net gain related to InterWell Health. Additionally, the FY 2022 basis for the 2023 outlook was adjusted for U.S. Provider Relief Funding. For FY 2023, special items include costs related to the FME25 program, the Humacyte investment remeasurement, the costs associated with the legal form conversion and effects from legacy portfolio optimization. For further details please see the reconciliation attached to the Press Release.
2 Net income attributable to shareholders of Fresenius Medical Care AG & Co. KGaA
In line with the Company’s disciplined financial policy, Fresenius Medical Care has already refinanced a bond of EUR 650 million, maturing in November 2023. The Company is using a mix of long-term bank financing at very attractive financing conditions as well as cash and short-term debt. Upcoming extraordinary cash inflows will enable the Company to further delever.
Revenue development supported by solid organic growth
Revenue decreased by 3% to EUR 4,936 million in the third quarter (+7% at constant currency, +7% organic).
Care Delivery revenue decreased by 4% to EUR 3,974 million (+6% at constant currency, +7% organic).
In Care Delivery U.S., revenue declined by 3% (+4% at constant currency, +5% organic). A negative exchange rate effect and a decrease in dialysis days was partially offset by organic growth, which was supported by a favorable impact from the value-based care business, reimbursement rate increases and a favorable payor mix. The annualization effect of COVID-19-related excess mortality in the late-stage CKD (Chronic Kidney Disease) and ESRD (End-Stage Renal Disease) population continues to weigh on same market treatment growth (-0.4%). Adjusted for the exit from less profitable acute care contracts same market treatment growth was at +0.2%.
In Care Delivery International, revenue declined by 7% (+14% at constant currency, +16% organic). A negative exchange rate effect and the impact of closed or sold clinics was partially offset by organic growth, which was driven by a significant effect of hyperinflation in various markets. Despite the annualization effect of COVID-19-related excess mortality, same market treatment growth was positive at 1.6%.
Care Enablement revenue declined by 3% to EUR 1,330 million (+5% at constant currency, +5% organic). The negative exchange rate effects have been partly offset by higher sales of in-center disposables, machines for chronic treatment and home hemodialysis products as well as higher average sales prices.
Within Inter-segment eliminations, revenue for products transferred between the operating segments at fair market value declined by 10% to EUR 368 million (-1% at constant currency).
In the first nine months, revenue was stable at EUR 14,466 million (+5% at constant currency, +5% organic). Care Delivery revenue was stable at EUR 11,602 million (+4% at constant currency, +5% organic), with stable revenue for Care Delivery U.S. (+2% at constant currency, +3% organic), and a revenue decline of 1% for Care Delivery International (+13% at constant currency, +14% organic). Care Enablement revenue was stable at EUR 3,965 million (+5% at constant currency, +5% organic). Inter-segment eliminations declined by 5% and amounted to EUR 1,101 million (stable at constant currency).3
3 The Company transfers products between segments at fair market value. The associated internal revenues and expenses and any remaining internally generated profit or loss for the product transfers are recorded within the operating segments initially, are eliminated upon consolidation and are included within “Inter-segment eliminations”.
Earnings development driven by productivity improvements and FME25 savings
Operating income decreased by 31% to EUR 324 million (-28% at constant currency), resulting in a margin of 6.6% (Q3 2022: 9.3%). Operating income excluding special items and U.S. Provider Relief Funding (PRF)1 increased by 14% to EUR 431 million (+20% at constant currency), resulting in a margin of 8.7% (Q3 2022: 7.4%).
Operating income in Care Delivery decreased by 34% to EUR 332 million (-29% at constant currency), resulting in a margin of 8.4% (Q3 2022: 12.1%). Operating income excluding special items and PRF1 increased by 11% to EUR 410 million (+17% at constant currency), resulting in a margin of 10.3% (Q3 2022: 9.0%). This was mainly driven by business growth, savings from the FME25 program and lower personnel expenses resulting from improved productivity. The operating income development was negatively impacted by lower income attributable to a non-recurring consent payment for certain pharmaceuticals, inflationary cost increases as well as by foreign currency translation.
Operating income in Care Enablement amounted to EUR -1 million (Q3 2022: EUR -26 million), resulting in a margin of -0.1% (Q3 2022: -1.9%). Operating income excluding special items increased by 197% to EUR 22 million (+217% at constant currency), resulting in a margin of 1.7% (Q3 2022: 0.5%). The improvement compared to the previous year’s quarter was mainly driven by increased volumes, improved pricing and savings from the FME25 program. These effects were partially offset by inflationary cost increases and negative foreign currency transaction effects.
Operating income for Corporate amounted to EUR -8 million (Q3 2022: EUR -7 million). Excluding special items, operating income amounted to EUR -2 million (Q3 2022: EUR -6 million).
In the first nine months, operating income decreased by 19% to EUR 942 million (-18% at constant currency), resulting in a margin of 6.5% (9M 2022: 8.1%). Excluding special items and PRF1, operating income increased by 13% to EUR 1,186 million (+14% at constant currency), resulting in a margin of 8.2% (9M 2022: 7.3%). In Care Delivery, operating income declined by 19% to EUR 1,001 million (-18% at constant currency), resulting in a margin of 8.6% (9M 2022: 10.6%). In Care Enablement, operating income decreased to EUR -24 million (9M 2022: EUR 33 million), resulting in a margin of -0.6% (9M 2022: 0.8%). Operating income for Corporate amounted to EUR -23 million (9M 2022: EUR -101 million).
Net income2 decreased by 63% to EUR 84 million (-61% at constant currency). Excluding special items and PRF1, net income2 remained stable at EUR 168 million (+5% at constant currency).
In the first nine months, net income2 declined by 42% to EUR 311 million (-41% at constant currency). Excluding special items and PRF1, net income2 increased by 3% to EUR 497 million (+5% at constant currency).
Basic earnings per share (EPS) decreased by 63% to EUR 0.29 (-61% at constant currency). EPS excluding special items and PRF1 remained stable at EUR 0.57 (+5% at constant currency).
In the first nine months, EPS declined by 42% to EUR 1.06 (-41% at constant currency). Excluding special items and PRF1, EPS increased by 3% to EUR 1.69 (+5% at constant currency).
Strong cash flow development
In the third quarter, Fresenius Medical Care generated EUR 760 million of operating cash flow (Q3 2022: EUR 658 million), resulting in a margin of 15.4% (Q3 2022: 12.9%). The increase in net cash provided by operating activities is the result of the change in certain working capital items, in particular due to the recoupment of advanced payments during 2022, which had been received under the U. S. Medicare Accelerated and Advance Payment Program in 2020.
In the first nine months, operating cashflow amounted to EUR 1,910 million (9M 2022: EUR 1,568 million), resulting in a margin of 13.2% (9M 2022: 10.9%).
Free cash flow4 amounted to EUR 626 million in the third quarter (Q3 2022:
EUR 501 million), resulting in a margin of 12.7% (Q3 2022: 9.8%). In the first nine months, Fresenius Medical Care generated free cash flow of EUR 1,480 million (9M 2022: EUR 1,082 million), resulting in a margin of 10.2% (9M 2022: 7.5%).
Outlook
The Company continues to expect for 2023 revenue to grow at a low to mid-single digit percentage rate (2022 basis: EUR 19,398 million).
Based on the earnings development for the first nine months of the year and solid business expectations for the remainder of the year, Fresenius Medical Care raises its earnings outlook for 2023. The Company now expects operating income to grow at a low-single digit percentage rate (2022 basis: EUR 1,540 million; previous target: remain flat or decline by up to a low-single digit percentage rate)5.
The Company’s target to achieve an operating income margin of 10 to 14% by 2025 remains unchanged.
4 Net cash provided by / used in operating activities, after capital expenditures, before acquisitions, investments, and dividends
5 Revenue and operating income, as referred to in the outlook, are both on a constant currency basis and excluding special items. Special items will be provided as separate KPI (“Revenue excluding special items”, “Operating income excluding special items”) to capture effects that are unusual in nature and have not been foreseeable or not foreseeable in size or impact at the time of giving guidance. These items are excluded to ensure comparability of the figures presented with the Company’s financial targets which have been defined excluding special items.
For FY 2022, special items included costs related to the FME25 program, the impact of the war in Ukraine, the impact of hyperinflation in Turkiye, the Humacyte investment remeasurement, and the net gain related to InterWell Health. Additionally, the basis (FY 2022) for the 2023 outlook was adjusted for Provider Relief Funding. For FY 2023, special items include costs related to the FME25 program, the Humacyte investment remeasurement, the costs associated with the legal form conversion and effects from legacy portfolio optimization. For further details please see the reconciliation attached to the Press Release.
Patients, clinics and employees
As of September 30, 2023, Fresenius Medical Care treated 341,793 patients in 4,014 dialysis clinics worldwide and had 123,106 employees (headcount) globally, compared to 130,295 employees as of September 30, 2022.
Conference call
Fresenius Medical Care will host a conference call to discuss the results of the third quarter on November 2, 2023 at 3:30 p.m. CET / 10:30 a.m. EDT. Details will be available on the Fresenius Medical Care website in the “Investors” section. A replay will be available shortly after the call.
Please refer to our statement of earnings included at the end of this news and to the attachments as separate PDF files for a complete overview of the results of the third quarter and first nine months of 2023. Our 6-K disclosure provides more details.
This release contains forward-looking statements that are subject to various risks and uncertainties. Actual results could differ materially from those described in these forward-looking statements due to various factors, including, but not limited to, changes in business, economic and competitive conditions, legal changes, regulatory approvals, impacts related to COVID-19, results of clinical studies, foreign exchange rate fluctuations, uncertainties in litigation or investigative proceedings, and the availability of financing. These and other risks and uncertainties are detailed in Fresenius Medical Care AG & Co. KGaA's reports filed with the U.S. Securities and Exchange Commission. Fresenius Medical Care AG & Co. KGaA does not undertake any responsibility to update the forward-looking statements in this release.
Implementation of measures as presented herein may be subject to information and consultation procedures with works councils and other employee representative bodies, as per local laws and practice. Consultation procedures may lead to changes on proposed measures.
Fresenius Medical Care, the world's leading provider of products and services for individuals with renal diseases, has appointed Craig Cordola (52) as new Management Board member for the globally operating Care Delivery segment. Cordola will start on January 1, 2024, as Chief Executive Officer of Care Delivery. He will be based in Waltham, Massachusetts.
Craig Cordola will succeed William (Bill) Valle (63) as part of a planned transition after Mr. Valle informed the Management Board of his intention to retire from the company at the end of 2023. Bill joined Fresenius Medical Care in 2009. He has been leading the globally operating Care Delivery segment since 2022. Previously, he served as CEO for North America since 2017. He has been a member of the Management Board since 2017. Prior to that, he was Executive Vice President responsible for the dialysis service business and vascular access business of Fresenius Medical Care North America from 2014 to 2017. Over the last decade he was responsible for the transformation of the U.S. business into a multi-dimensional integrated kidney care provider.
Craig Cordola is currently Executive Vice President of Ascension Capital, where he is responsible for strategic investments. Previously, he was Executive Vice President and Chief Operating Officer for Ascension. Prior to joining Ascension in 2017, Mr. Cordola held several senior executive and leadership positions at Memorial Hermann Health System in Houston, Texas. He is a Fellow of the American College of Healthcare Executives and holds a degree in Psychology from The University of Texas at Austin. He also earned dual degrees with a Master of Healthcare Administration (MHA) and a Master of Business Administration (MBA) from the University of Houston-Clear Lake.
Michael Sen, Chairman of the Supervisory Board of Fresenius Medical Care Management AG, said: “We are delighted to have Craig Cordola join the Management Board of Fresenius Medical Care as the leader of the global Care Delivery organization. Craig has a wealth of knowledge and experience managing large healthcare systems and organizations. With this expertise, he will be a crucial player in the Management Board in implementing Fresenius Medical Care's turnaround and focusing on patient care.” Michael Sen added: “On behalf of the entire Supervisory Board, I would like to express my sincere appreciation to Bill for his excellent work over the past years, his passion for the well-being of patients and his contribution to the development of Fresenius Medical Care. We wish him all the best for his retirement.”
Helen Giza, CEO and Chair of the Management Board, said: “Craig joins Fresenius Medical Care with many years of proven track record of operational management experience and successfully driving profitable business growth in different companies in the U.S. healthcare services industry. He will be a valuable member of my leadership team continuing our turnaround and transformation while leading Care Delivery into its next chapter. I would like to thank Bill for his many years of leadership and remarkable contributions to Fresenius Medical Care throughout his tenure with us, his passion for our patients and employees shines through in everything he does. I wish him well in his retirement.”
Craig Cordola said: “I am excited to join Fresenius Medical Care, the global leader in kidney care and leading healthcare brand across this industry. I cannot wait to partner with its leaders – and all of its team members – as we transform our organization to continue to serve patients and communities that suffer from renal disease.”
This release contains forward-looking statements that are subject to various risks and uncertainties. Actual results could differ materially from those described in these forward-looking statements due to various factors, including, but not limited to, changes in business, economic and competitive conditions, legal changes, regulatory approvals, impacts related to the COVID-19 pandemic results of clinical studies, foreign exchange rate fluctuations, uncertainties in litigation or investigative proceedings, and the availability of financing. These and other risks and uncertainties are detailed in Fresenius Medical Care AG & Co. KGaA's reports filed with the U.S. Securities and Exchange Commission. Fresenius Medical Care AG & Co. KGaA does not undertake any responsibility to update the forward-looking statements in this release.
- Company-wide research offers novel pathways for enhancing kidney patient care using data-driven insights and real-world evidence
Fresenius Medical Care, the world's leading provider of products and services for individuals with renal diseases, today announced the presentation of over 60 company-affiliated research abstracts at the American Society of Nephrology’s (ASN) Kidney Week 2023 taking place November 2-5 in Philadelphia.
“The scientific and medical breadth of research presented this year highlights the power of Fresenius Medical Care’s global scope and scale in creating data-driven insights that have the potential to improve patient care and outcomes,” said Dr. Frank Maddux, Global Chief Medical Officer and Member of the Management Board for Fresenius Medical Care. “Our presentations cover key topics including advances in machine learning and artificial intelligence in kidney care, using big data and real-world evidence to drive insights, advancing home dialysis, and research around hemodiafiltration (HDF) and hemodialysis (HD) therapies. We look forward to sharing our insights at this year’s conference.”
Scientific and medical experts from across Fresenius Medical Care will present research related to many important topics in kidney disease care. A link to all Fresenius Medical Care-affiliated presentations can be found on our company’s website: https://fmcna.com/ASN-2023/. Highlights of this year’s presentations for the medical congress include:
- Artificial Intelligence and Data Science:
- Impact of Nationwide Utilization of a Machine Learning Model to Identify Home Therapy Candidates (November 2, 10:00 AM – 12:00 PM): Education on a machine learning predictive model which identifies in-center hemodialysis patients who would be good candidates for home therapy.
- Comparative Prognostic Accuracy of Vascular Access Flow and Artificial Intelligence (AI)-Based Arteriovenous Fistula (AVF) Failure Risk Score (November 3, 10:00 AM – 12:00 PM): Presenting a risk score which accurately and reproducibly predicts AVF failure within 3 months that may offer a cheaper and automated alternative to Qa measurement.
- Data-Driven Global Insights:
- Apollo DB: Characteristics of a Global Dialysis Database Across Major World Regions (November 2, 10:00 AM – 12;00 PM): Capturing data from over 40 countries, Apollo DB is an anonymized dialysis database that combines and harmonizes data from a global provider for research and quality improvement activities.
- Characteristics of Global Dialysis Data from Multiple Providers in the New MONitoring Dialysis Outcomes (MONDO) Dataset (November 2, 10:00 AM – 12:00 PM): Research captured from 20 years of longitudinal patient data contributed to the new MONDO dataset, the most robust global dialysis dataset in the world.
- Creatinine Clearance Predicts Longitudinal Phosphate Levels Irrespective of Achieved Urea Kt/V: A Peritoneal Dialysis-MONDO Analysis (November 4, 10:00 AM – 12:00 PM): Experts present a study designed to evaluate if CrCl predicts longitudinal PO4 irrespective of achieved Kt/V.
- Hemodiafiltration (HDF) and Hemodialysis (HD) Research:
- Relative Blood Volume Monitoring Using Crit-Line and Hospital Admissions: A Retrospective Analysis of over 25,000 Patients Across 330 Dialysis Clinics (November 2, 10:00 AM – 12:00 PM): Presenting a comparison of hospital admissions among Fresenius Kidney Care (FKC) clinics with high utilization of RBV and propensity score matched (PSM) clinics not using RBV.
- Real-World Effectiveness of Hemodialysis Modalities (November 2, 10:00 AM – 12:00 PM): A late-breaking abstract that provides real world evidence demonstrating comparison of patients treated with hemodialysis vs hemodiafiltration.
- Dialyzer Performance and Associations in Self-Reported Pruritis and Fatigue: Results from the eMPORA III Trial (November 3, 10:00 AM – 12:00 PM): eMPORA III was a multi-center crossover trial with 4-week randomized treatment periods comparing dialyzer performance (FX CorAL 600 vs two comparable high-flux dialyzers) in post-dilution online hemodiafiltration (HDF).
- Psychometric Validation of the CONVINCE Inter- and Intradialytic Symptoms Questionnaire (November 3, 10:00 AM – 12:00 PM): The CONVINCE Scientific Committee and CONVINCE Investigators present the survey developed based on the KDQOL symptoms scale with an adapted recall period of 7 days for interdialytic symptoms and extended inter- and intradialytic (IDS) items based on literature review and patient interviews.
This release contains forward-looking statements that are subject to various risks and uncertainties. Actual results could differ materially from those described in these forward-looking statements due to various factors, including, but not limited to, changes in business, economic and competitive conditions, legal changes, regulatory approvals, impacts related to the COVID-19 pandemic results of clinical studies, foreign exchange rate fluctuations, uncertainties in litigation or investigative proceedings, and the availability of financing. These and other risks and uncertainties are detailed in Fresenius Medical Care AG & Co. KGaA's reports filed with the U.S. Securities and Exchange Commission. Fresenius Medical Care AG & Co. KGaA does not undertake any responsibility to update the forward-looking statements in this release.
- Powerful Cloud-Based Data Asset to Drive Improvements in Care Quality and Patient Outcomes as the Largest Multinational, Longitudinal Database of its kind
- Achievement Part of Company’s Long-Term AI Strategy and Ongoing Digital Transformation Efforts
Fresenius Medical Care, the world's leading provider of products and services for individuals with renal diseases, today announced the phase-one completion of the company’s first anonymized global dialysis dataset—coined the Apollo database project—the foundation of the company’s long-term AI aspirations. Intended to advance patient care quality and outcomes by making kidney disease care more personalized and precise, the database provides a highly sophisticated view into the clinical care provided to more than 540 thousand dialysis patients, the largest multinational, longitudinal database of its kind.
“Artificial intelligence is only as good as the data that powers it,” says Frank Maddux, MD, Global Chief Medical Officer and Member of the Management Board. “The Apollo database is not only helping advance our understanding of kidney disease and dialysis therapies through data-driven insights. It also provides data that is high quality, relevant and timely, three vital data attributes that are crucial to achieving AI aspirations at scale.”
Phase one of the Apollo Database project harmonizes data from across the company’s global clinical systems into the cloud, aggregating data from 40 countries across 6 continents on more than 350 patient treatment parameters. It includes information from more than 540 thousand dialysis patients, more than 140 million dialysis treatments, and more than 34 million laboratory assessments.
The database provides full anonymization of data and a streamlined pathway for global analytics all while adhering to the complex set of global, regional and local privacy requirements, including HIPAA and GDPR.
“The data created across Fresenius Medical Care’s global clinical footprint is unmatched in its breadth and depth, and is one of the company’s greatest competitive advantages,” said Stuart McGuigan, Global Chief Information Officer of Fresenius Medical Care. “Reimagining our digital infrastructure has been a key part of our organizational transformation, and the achievement of the Apollo Database project is an important benchmark in not just our digital transformation, but in our long-term AI strategy.”
The Apollo Database project will be featured in numerous research presentations by Fresenius Medical Care experts at this year’s American Society of Nephrology Kidney Week conference—one of the world’s largest and most influential gatherings of kidney disease physicians and experts—happening November 2-5 in Philadelphia, Pennsylvania in the U.S.
“Dialysis care generates a large amount of data that can be used for secondary purposes, but multinational datasets are scarce due to the fundamental need for adherence to varying complex data protection regulations around the world, as well as the challenges in harmonization of data from different clinical systems,” said Len Usvyat, PhD, Head of Clinical Advanced Analytics for Fresenius Medical Care. “This important data tool increases the speed and robustness of the company’s analytical capabilities and provides greater consistency in generating data-driven clinical insights. The knowledge gained from these efforts have the potential to improve not just the practice of medicine, but more importantly the quality of life for people with kidney disease.”
The project is coordinated by the Global Medical Office in collaboration with the company’s Digital Technology & Innovation, Care Delivery and Care Enablement teams. The project is already powering more than 15 clinical improvement projects, such as a global feasibility assessment of the expanded use of an Anemia Control Model, an artificial intelligence model being used in many countries to optimize use of erythropoietin stimulating agents and iron therapies in dialysis patients.
This release contains forward-looking statements that are subject to various risks and uncertainties. Actual results could differ materially from those described in these forward-looking statements due to various factors, including, but not limited to, changes in business, economic and competitive conditions, legal changes, regulatory approvals, impacts related to the COVID-19 pandemic results of clinical studies, foreign exchange rate fluctuations, uncertainties in litigation or investigative proceedings, and the availability of financing. These and other risks and uncertainties are detailed in Fresenius Medical Care AG & Co. KGaA's reports filed with the U.S. Securities and Exchange Commission. Fresenius Medical Care AG & Co. KGaA does not undertake any responsibility to update the forward-looking statements in this release.
Fresenius Medical Care, the world’s leading provider of products and services for individuals with renal diseases, today announced the U.S. Food and Drug Administration (FDA) 510(k) clearance for Versi®HD with GuideMe Software, a completely reinvented self-guided interface for the company’s VersiHD chronic home hemodialysis (HHD) system. VersiHD with GuideMe Software aims to transform the experience of HHD for patients and nurses.
As the established leader in HHD, NxStage from Fresenius Medical Care, draws on its experience of over 30 million patient treatments at home over nearly two decades, serving patients in more than 45,000 homes with a broad diversity of water sources across the U.S.
VersiHD with GuideMe Software provides graphical walk-through guidance that aims to enhance ease of use and confidence for both patients and nurses. It is designed to improve patient training time, ease the transition to home, and make the training experience easier for new users.
“With over 13,000 HHD patients in the U.S. alone, we are excited to amplify the innovation NxStage offers with advanced technologies, like VersiHD with GuideMe Software,” said Dr. Katarzyna Mazur-Hofsäß, CEO of Fresenius Medical Care’s Care Enablement segment. “We are committed to providing cutting-edge solutions to improve the health and well-being of dialysis patients by challenging the standard of home dialysis with the design of industry-leading products.”
“We expect VersiHD with GuideMe Software to further simplify home hemodialysis for patients during training and at home,” said Dr. Brigitte Schiller, Senior Vice President, Medical Officer, Home Therapies at Fresenius Medical Care. “As a result of this software upgrade, patients and their care partners will have additional support to be confident with the therapy at home. VersiHD with GuideMe Software will support Fresenius Medical Care’s mission of advancing access to home therapies to more patients.”
NxStage has had many significant innovations over the years guided by patient input starting with the first portable HHD machine, NxStage System One™, in 2005 followed by the introduction of the PureFlow™ DI Water Purification System in 2006. Nx2me Connected Health®, the first Connected Health platform for HHD followed in 2014. The NxStage System One was the first machine to receive FDA clearance for Nocturnal HHD indication in 2014 and also the first to receive FDA clearance for Solo HHD indication in 2017. In 2017, NxStage introduced the VersiHD touchscreen cycler. Over 95% of HHD patients in the U.S. use the NxStage HHD system.
VersiHD with GuideMe Software will initially be available in selected markets in 2023. Existing VersiHD systems will be upgradeable to GuideMe Software. For more information about VersiHD with GuideMe Software, please visit www.nxstage.com/hcp/versihdguideme.
Despite the health benefits that home hemodialysis may provide to those with chronic kidney disease, this form of therapy is not for everyone. The reported benefits of home hemodialysis may not be experienced by all patients. The risks associated with hemodialysis treatments in any environment include, but are not limited to, high blood pressure, fluid overload, low blood pressure, heart-related issues, and vascular access complications. The medical devices used in hemodialysis therapies may add additional risks including air entering the bloodstream and blood loss due to clotting or accidental disconnection of the blood tubing set. Certain risks are unique to the home. Treatments at home are done without the presence of medical personnel and on-site technical support. Patients and their partners must be trained on what to do and how to get medical or technical help if needed.
© 2023 Fresenius Medical Care. All Rights Reserved. Fresenius Medical Care, NxStage, the triangle logo, VersiHD, PureFlow and Nx2me are trademarks of Fresenius Medical Care Holdings, Inc. or its affiliated companies. All other trademarks are the property of their respective owners.
Fresenius Medical Care is the world's leading provider of products and services for individuals with renal diseases of which around 3.9 million patients worldwide regularly undergo dialysis treatment. Through its network of 4,050 dialysis clinics, Fresenius Medical Care provides dialysis treatments for approximately 344,000 patients around the globe. Fresenius Medical Care is also the leading provider of dialysis products such as dialysis machines or dialyzers. Fresenius Medical Care is listed on the Frankfurt Stock Exchange (FME) and on the New York Stock Exchange (FMS).
For more information visit the Company’s website at www.freseniusmedicalcare.com.
Disclaimer:
This release contains forward-looking statements that are subject to various risks and uncertainties. Actual results could differ materially from those described in these forward-looking statements due to various factors, including, but not limited to, changes in business, economic and competitive conditions, legal changes, regulatory approvals, impacts related to the COVID-19 pandemic results of clinical studies, foreign exchange rate fluctuations, uncertainties in litigation or investigative proceedings, and the availability of financing. These and other risks and uncertainties are detailed in Fresenius Medical Care AG & Co. KGaA's reports filed with the U.S. Securities and Exchange Commission. Fresenius Medical Care AG & Co. KGaA does not undertake any responsibility to update the forward-looking statements in this release.
- Organic growth accelerated in the second quarter in Care Enablement and Care Delivery including sequentially stable treatment volumes in the U.S.
- Execution on turnaround plan translates into visible productivity improvements in Care Delivery achieving a Q2 margin at the lower end of the 2025 target margin band
- Savings resulting from FME25 transformation program fully on track
- Successful execution on portfolio optimization strategy
- Legal form conversion to a German Stock Corporation approved by shareholders
- FY 2023 operating income guidance range narrowed
Helen Giza, Chief Executive Officer of Fresenius Medical Care, said: “The second quarter makes evident that the execution against our strategic plan is fully on track. We are executing on our portfolio optimization, continuing to deliver on our FME25 program and are accelerating our turnaround activities. As expected, we have seen a stabilization of the labor market and of the inflationary environment. Our measures to increase productivity, supported by the targeted clinic closures, are driving a positive development. This gives us the confidence to narrow our operating income guidance range to the upper part for the year.”
Key figures (IFRS®, unaudited)
|
|
Q2 2023 |
Q2 2022 |
Growth |
Growth |
H1 2023 |
H1 2022 |
Growth |
Growth |
|
|
EUR m |
EUR m |
yoy |
yoy, cc |
EUR m |
EUR m |
yoy |
yoy, cc |
|
Revenue |
4,825 |
4,757 |
+1 % |
+6 % |
9,529 |
9,305 |
+2 % |
+4 % |
|
|
|
|
|
|
|
|
|
|
|
Operating income |
357 |
341 |
+5 % |
+5 % |
618 |
688 |
-10 % |
-11 % |
|
excl. special items and PRF1 |
401 |
284 |
+41% |
+44% |
755 |
675 |
+12% |
+11% |
|
|
|
|
|
|
|
|
|
|
|
Net income2 |
140 |
147 |
-5 % |
-4 % |
227 |
305 |
-26 % |
-26 % |
|
excl. special items and PRF1 |
175 |
116 |
+51% |
+54% |
329 |
313 |
+5% |
+5% |
|
|
|
|
|
|
|
|
|
|
|
Basic EPS (EUR) |
0.48 |
0.50 |
-5 % |
-4 % |
0.77 |
1.04 |
-26 % |
-26 % |
|
excl. special items and PRF1 |
0.59 |
0.39 |
+51% |
+54% |
1.12 |
1.07 |
+5% |
+4% |
|
|
|
|
|
|
|
|
|
|
yoy = year-on-year, cc = at constant currency, EPS = earnings per share
Successful execution against the strategic plan
Fresenius Medical Care has continuously advanced its structural change. At the beginning of the year the new operating model was implemented along with the corresponding new financial reporting. The simplification of the governance structure with the change of the legal form is thus the remaining structural adjustment to be realized. An important milestone has been achieved in this respect at the Extraordinary General Meeting on July 14, 2023, where 99.88% of the voting shareholders approved the conversion of Fresenius Medical Care from the legal form of a partnership limited by shares (Kommanditgesellschaft auf Aktien, KGaA) into a German stock corporation (Aktiengesellschaft, AG).
In parallel, the Company continuously executes on its operational efficiency and turnaround plans. In the second quarter, the FME25 transformation program delivered EUR 75 million of additional savings. Fresenius Medical Care is fully on track to achieve sustainable savings of EUR 250 to 300 million by year end 2023 and EUR 650 million by year end 2025.
In addition to generating efficiencies and improving productivity, Fresenius Medical Care is advancing the optimization of its portfolio. The announced strategic divestments of clinic networks in Sub-Saharan Africa and Hungary demonstrate progress against the Company’s execution plan. The outlined examples are part of the overall portfolio optimization strategy to exit non-core and dilutive assets, against which Fresenius Medical Care executes. The resulting cash proceeds will be used towards deleveraging – in line with Fresenius Medical Care’s disciplined financial policy.
1 For FY 2022, special items included costs related to the FME25 program, the impact of the war in Ukraine, the impact of hyperinflation in Turkiye, the Humacyte investment remeasurement and the net gain related to InterWell Health. Additionally, the FY 2022 basis for the 2023 outlook was adjusted for U.S. Provider Relief Funding. For FY 2023, special items include costs related to the FME25 program, the Humacyte investment remeasurement, the costs associated with the legal form conversion and effects from legacy portfolio optimization. For further details please see the reconciliation attached to the Press Release.
2 Net income attributable to shareholders of Fresenius Medical Care AG & Co. KGaA
Earnings development excluding special items driven by FME25 savings and productivity improvements
Revenue increased by 1% to EUR 4,825 million in the second quarter (+6% at constant currency, +6% organic).
Care Delivery revenue increased by 1% to EUR 3,873 million (+6% at constant currency, +6% organic).
In Care Delivery U.S., revenue growth of 2% (+4% at constant currency, +4% organic) was mainly driven by organic growth, which was supported by a favorable impact from the value-based care business, reimbursement rate increases and a favorable payor mix. This was partially offset by a negative exchange rate effect. The annualization effect of COVID-19-related excess mortality in the late-stage CKD (Chronic Kidney Disease) and ESRD (End-Stage Renal Disease) population continues to weigh on same market treatment growth (-0.1%) – corresponding to the mid-point of the Company’s outlined expectations.
In Care Delivery International, revenue remained stable (+14% at constant currency, +15% organic). Organic growth, which was supported by the effect of hyperinflation in various markets, was offset by a negative exchange rate effect and the impact of closed or sold clinics. Despite the annualization effect of COVID-19-related excess mortality, same market treatment growth was positive at 0.9%.
Care Enablement revenue remained stable and amounted to EUR 1,325 million (+6% at constant currency, +6% organic). Higher sales of machines for chronic treatment, critical care products and home hemodialysis products as well as increased average sales prices were mostly offset by a negative exchange rate effect.
Within Inter-segment eliminations, revenue for products transferred between the operating segments at fair market value decreased by 3% to EUR 373 million (+3% at constant currency; Q2 2022: EUR 383 million).3
In the first half, revenue increased by 2% to EUR 9,529 million (+4% at constant currency, +4% organic). Care Delivery revenue increased by 2% to 7,628 million (+3% at constant currency, +4% organic), with both Care Delivery U.S. and Care Delivery International growing by 2% (U.S.: +1% at constant currency, +2% organic; International: +13% at constant currency, +14% organic). Care Enablement revenue increased by 2% to EUR 2,635 million (+5% at constant currency, +5% organic). Inter-segment eliminations decreased by 2% and amounted to EUR 734 million (stable at constant currency; H1 2022: EUR 750 million).
3 The Company transfers products between segments at fair market value. The associated internal revenues and expenses and any remaining internally generated profit or loss for the product transfers are recorded within the operating segments initially, are eliminated upon consolidation and are included within “Inter-segment eliminations”.
Operating income increased by 5% to EUR 357 million (+5% at constant currency), resulting in a margin of 7.4% (Q2 2022: 7.2%). Operating income excluding special items and U.S. Provider Relief Funding (PRF)1 increased by 41% to EUR 401 million (+44% at constant currency), resulting in a margin of 8.3% (Q2 2022: 6.0%).
Operating income in Care Delivery decreased by 11% to EUR 384 million (-10% at constant currency), resulting in a margin of 9.9% (Q2 2022: 11.3%). Operating income excluding special items and PRF1 increased by 40% to EUR 402 million (+42% at constant currency), resulting in a margin of 10.4% (Q2 2022: 7.5%). This was mainly driven by business growth, lower personnel expenses resulting from improved productivity, and savings from the FME25 program.
Operating income in Care Enablement amounted to EUR 2 million (Q2 2022: EUR -11 million), resulting in a margin of 0.1% (Q2 2022: -0.8%). Operating income excluding special items increased by 533% to EUR 19 million (+601% at constant currency), resulting in a margin of 1.4% (Q2 2022: 0.2%). The improvement compared to the previous year’s quarter was mainly driven by increased volumes, improved pricing and savings from the FME25 program. These effects were partially offset by inflationary cost increases and a negative impact from foreign currency transaction.
Operating income for Corporate amounted to EUR -25 million (Q2 2022: EUR -84 million). Excluding special items, operating income amounted to EUR -16 million (Q2 2022: EUR -9 million).
In the first half, operating income decreased by 10% to EUR 618 million (-11% at constant currency), resulting in a margin of 6.5% (H1 2022: 7.4%). Excluding special items and PRF1, operating income increased by 12% to EUR 755 million (+11% at constant currency), resulting in a margin of 7.9% (H1 2022: 7.2%). In Care Delivery, operating income declined by 8% to EUR 669 million (-10% at constant currency), resulting in a margin of 8.8% (H1 2022: 9.8%). In Care Enablement, operating income decreased to EUR -23 million (H1 2022: EUR 59 million), resulting in a margin of -0.9% (H1 2022: 2.3%). Operating income for Corporate amounted to EUR -15 million (H1 2022: -94 million).
Net income2 decreased by 5% to EUR 140 million (-4% at constant currency). Excluding special items and PRF1, net income2 increased by 51% to EUR 175 million (+54% at constant currency).
In the first half, net income2 declined by 26% to EUR 227 million (-26% at constant currency). Excluding special items and PRF1, net income2 increased by 5% to EUR 329 million (+5% at constant currency).
Basic earnings per share (EPS) decreased by 5% to EUR 0.48 (-4% at constant currency). EPS excluding special items and PRF1 increased by 51% to EUR 0.59 (+54% at constant currency).
In the first half, EPS declined by 26% to EUR 0.77 (-26% at constant currency). Excluding special items and PRF1, EPS increased by 5% to EUR 1.12 (+4% at constant currency).
Strong cash flow development
In the second quarter, Fresenius Medical Care generated EUR 1,007 million of operating cash flow (Q2 2022: EUR 751 million), resulting in a margin of 20.9% (Q2 2022: 15.8%). The increase was mainly driven by the recoupment of advanced payments during 2022, which had been received in the U.S. under the Medicare Accelerated and Advance Payment Program in 2020, as well as by seasonality of invoicing.
In the first half, operating cashflow amounted to EUR 1,150 million (H1 2022: EUR 910 million), resulting in a margin of 12.1% (H1 2022: 9.8%).
Free cash flow2 amounted to EUR 852 million in the second quarter (Q2 2022: EUR 582 million), resulting in a margin of 17.7% (Q2 2022: 12.2%). In the first half, Fresenius Medical Care generated free cash flow of EUR 854 million (H1 2022: EUR 581 million), resulting in a margin of 9.0% (H1 2022: 6.2%).
4 Net cash provided by / used in operating activities, after capital expenditures, before acquisitions, investments, and dividends
Outlook
The Company continues to expect for 2023 revenue to grow at a low to mid-single digit percentage rate (2022 basis: EUR 19,398 million).
Based on the earnings development for the first half of the year, Fresenius Medical Care narrows its operating income target range for 2023. The Company now expects operating income to remain flat or decline by up to a low-single digit percentage rate (2022 basis: EUR 1,540 million; previous target: remain flat or decline by up to a high-single digit percentage rate)5.
The Company’s target to achieve an operating income margin of 10 to 14% by 2025 remains unchanged.
5 Revenue and operating income, as referred to in the outlook, are both on a constant currency basis and excluding special items. Special items will be provided as separate KPI (“Revenue excluding special items”, “Operating income excluding special items”) to capture effects that are unusual in nature and have not been foreseeable or not foreseeable in size or impact at the time of giving guidance. These items are excluded to ensure comparability of the figures presented with the Company’s financial targets which have been defined excluding special items.
For FY 2022, special items included costs related to the FME25 program, the impact of the war in Ukraine, the impact of hyperinflation in Turkiye, the Humacyte investment remeasurement, and the net gain related to InterWell Health. Additionally, the basis (FY 2022) for the 2023 outlook was adjusted for Provider Relief Funding. For FY 2023, special items include costs related to the FME25 program, the Humacyte investment remeasurement, the costs associated with the legal form conversion and effects from legacy portfolio optimization. For further details please see the reconciliation attached to the Press Release.
Patients, clinics and employees
As of June 30, 2023, Fresenius Medical Care treated 344,086 patients in 4,050 dialysis clinics worldwide and had 124,295 employees (headcount) globally, compared to 130,448 employees as of June 30, 2022.
Conference call
Fresenius Medical Care will host a conference call to discuss the results of the second quarter and first half of 2023 on August 2, 2023 at 3:30 p.m. CEST / 9:30 a.m. EDT. Details will be available on the Fresenius Medical Care website in the “Investors” section. A replay will be available shortly after the call.
Please refer to our statement of earnings included at the end of this news and to the attachments as separate PDF files for a complete overview of the results of the second quarter and first half of 2023. Our 6-K disclosure provides more details.
This release contains forward-looking statements that are subject to various risks and uncertainties. Actual results could differ materially from those described in these forward-looking statements due to various factors, including, but not limited to, changes in business, economic and competitive conditions, legal changes, regulatory approvals, impacts related to COVID-19, results of clinical studies, foreign exchange rate fluctuations, uncertainties in litigation or investigative proceedings, and the availability of financing. These and other risks and uncertainties are detailed in Fresenius Medical Care AG & Co. KGaA's reports filed with the U.S. Securities and Exchange Commission. Fresenius Medical Care AG & Co. KGaA does not undertake any responsibility to update the forward-looking statements in this release.
Implementation of measures as presented herein may be subject to information and consultation procedures with works councils and other employee representative bodies, as per local laws and practice. Consultation procedures may lead to changes on proposed measures.
- Extraordinary General Meeting approves conversion of Fresenius Medical Care’s legal form
- Several advantages including faster and more agile decision-making
- Michael Sen elected as Chair of new Supervisory Board
- Conversion expected to be completed by the end of 2023
Fresenius Medical Care, the world’s leading provider of products and services for individuals with renal diseases, held an Extraordinary General Meeting (EGM) today. The Company’s shareholders approved all agenda items with large majority, including the conversion of Fresenius Medical Care from the legal form of a partnership limited by shares (Kommanditgesellschaft auf Aktien, KGaA) into a German stock corporation (Aktiengesellschaft, AG), and elected the four shareholder representatives on the Supervisory Board of the new Fresenius Medical Care AG. In its constituting meeting following the EGM, the new Supervisory Board elected Michael Sen as its Chair.
Michael Sen, Chair of the Supervisory Board of Fresenius Medical Care AG, said: “Our shareholders’ vote today clearly is a sign of confidence showing that we are on the right track with Fresenius and with Fresenius Medical Care. As the Chair of the Supervisory Board, I am delighted to lead this new Supervisory Board consisting of highly qualified individuals. Their diverse backgrounds and experiences will provide a great balance between continuity and fresh perspectives. I look forward to working with this great team. Furthermore, I would like to express my sincere appreciation to the long-standing Chair of the Supervisory Board, Dr Dieter Schenk, who has supported and guided the company for almost three decades. My gratitude also goes to Rolf Classon, Dr Dorothea Wenzel and Professor Dr Gregor Zünd whose mandates as Supervisory Board members will end once the conversion is completed.”
Helen Giza, CEO of Fresenius Medical Care AG, said: “Today’s decision of our shareholders to convert Fresenius Medical Care into a German stock corporation opens a new chapter in the development of the Company. I strongly believe that following the conversion, the decision-making processes will be accelerated. Therefore, we will be more agile in our efforts in unlocking value as the leading kidney care Company. The role of our free float shareholders will also be particularly strengthened. I’m excited about collaborating with our new Supervisory Board and leading Fresenius Medical Care into a successful future, together with my team and our committed employees around the world.”
Following the conversion into a German stock corporation, Fresenius Medical Care will have a standard German two-tier Board system that is familiar to shareholders and in line with widely recognized corporate governance practices. The co-determined Supervisory Board will in future consist of twelve members. At today’s EGM, Shervin J. Korangy, Dr Marcus Kuhnert, Gregory Sorensen, M.D. and Pascale Witz were elected members of the new Supervisory Board. In addition to them, Fresenius SE & Co. KGaA (Fresenius), which holds approx. 32.2 percent of the ordinary share capital, appoints two members to the new Supervisory Board: its CEO Michael Sen, who has been elected as Chair of the new Supervisory Board, as well as its CFO Sara Hennicken. This is a testament to Fresenius’ close relationship with Fresenius Medical Care and its continued commitment to the Company. The other six members of the new Supervisory Board will be elected by the employees at a later point in time. The new Supervisory Board will perform all supervisory functions, including strategy review, management appointments, remuneration, approval of important management decisions and audit once the conversion becomes effective.
Today, the new Supervisory Board of Fresenius Medical Care AG also formally appointed the Management Board of the future Fresenius Medical Care AG, which consists of the members of the existing Management Board of Fresenius Medical Care Management AG.
In addition to the simplification of the corporate governance, the conversion into a German stock corporation provides further advantages. The simplified structure will, for instance, lead to a more efficient and faster decision-making as it allows for a clearer focus on the interests of the Fresenius Medical Care group and frees up management resources. Fresenius Medical Care will also have greater flexibility concerning its financial strategy. Furthermore, the role of free float shareholders will be strengthened as their influence on the composition of Fresenius Medical Care’s management increases.
Given the approval of all agenda items at today’s EGM, all necessary administrative, compliance and regulatory steps will now be initiated. The entire process of the conversion of Fresenius Medical Care into a German stock corporation is expected to be completed by the end of 2023. Until completion, the current corporate governance structure and the corresponding corporate bodies, including the current Supervisory Boards, remain in place.
This release contains forward-looking statements that are subject to various risks and uncertainties. Actual results could differ materially from those described in these forward-looking statements due to various factors, including, but not limited to, changes in business, economic and competitive conditions, legal changes, regulatory approvals, impacts related to the COVID-19 pandemic results of clinical studies, foreign exchange rate fluctuations, uncertainties in litigation or investigative proceedings, and the availability of financing. These and other risks and uncertainties are detailed in Fresenius Medical Care AG & Co. KGaA's reports filed with the U.S. Securities and Exchange Commission. Fresenius Medical Care AG & Co. KGaA does not undertake any responsibility to update the forward-looking statements in this release.
Under the U.S. Securities Act of 1933, as amended (the “Securities Act”), this press release may be deemed to be offering material of Fresenius Medical Care AG & Co. KGaA (“FME”). FME has filed a registration statement on Form F-4 under the Securities Act with the U.S. Securities and Exchange Commission (the “SEC”), including an information statement/prospectus constituting a part thereof. FME SHAREHOLDERS ARE URGED TO READ THE REGISTRATION STATEMENT AND ANY OTHER RELEVANT DOCUMENTS FILED OR THAT WILL BE FILED WITH THE SEC, INCLUDING THE INFORMATION STATEMENT/PROSPECTUS THAT IS PART OF THE REGISTRATION STATEMENT, AS THEY BECOME AVAILABLE, BECAUSE THEY CONTAIN OR WILL CONTAIN IMPORTANT INFORMATION ABOUT THE PROPOSED CONVERSION DESCRIBED THEREIN. The final information statement/prospectus has been distributed to FME shareholders. Shareholders may obtain a free copy of the disclosure documents and other documents filed by FME with the SEC at the SEC’s website at www.sec.gov or from Fresenius Medical Care AG & Co. KGaA, Attention: Investor Relations, Else-Kröner-Straße 1, 61352 Bad Homburg v.d.H., Germany.
Fresenius Medical Care, the world's leading provider of products and services for individuals with renal diseases has appointed Martin Fischer (46) as Chief Financial Officer as of October 1, 2023. He will succeed Helen Giza who was appointed as Chief Executive Officer and Chair of the Management Board in December 2022 and continues to serve as acting Chief Financial Officer, until her successor will join. Martin Fischer will be based in Bad Homburg, Germany and will assume responsibility for the Global Finance Organization of Fresenius Medical Care. Upon effectiveness of the Company’s proposed change of form from KGaA to German stock corporation, Martin Fischer will become a member of the Management Board of Fresenius Medical Care AG.
Martin Fischer has been Head of Finance for Siemens Healthineers Diagnostics Division based in Tarrytown, NY, U.S. since 2019. Previously, he headed the Board Office and Organizations function for Siemens Healthineers after leading the business plan and operating model development for the company’s initial public offering in March 2018. Prior to that, Fischer held a number of key international operational and finance positions in healthcare within Siemens AG. Martin Fischer holds a degree in business informatics from the Reutlingen University of Applied Sciences and an MBA from Friedrich Alexander University in Nuremberg. He completed the Chief Financial Officer Program at Columbia Business School in New York, USA.
Michael Sen, Chairman of the Supervisory Board of Fresenius Medical Care Management AG, says: "With Martin Fischer's appointment, we are strengthening a vital function in the Management Board of Fresenius Medical Care. Martin Fischer has a deep understanding of the international healthcare market, both from the U.S. perspective and out of Germany. That's a decisive advantage in getting the company back on track."
Helen Giza, CEO and Chair of the Management Board, said: "Martin Fischer has proven that he can successfully drive fundamental change in organizations. In our organizational transformation and turnaround management, we will benefit from his finance and healthcare expertise. Martin will be an important contributor to the execution of our strategy in unlocking value as the leading kidney care company."
Martin Fischer said: “There are great challenges ahead of us. I am convinced of the company's potential and look forward to realizing it together with the global team of Fresenius Medical Care. I am excited to bring my experience and expertise to support the company’s transformation.”
This release contains forward-looking statements that are subject to various risks and uncertainties. Actual results could differ materially from those described in these forward-looking statements due to various factors, including, but not limited to, changes in business, economic and competitive conditions, legal changes, regulatory approvals, impacts related to the COVID-19 pandemic results of clinical studies, foreign exchange rate fluctuations, uncertainties in litigation or investigative proceedings, and the availability of financing. These and other risks and uncertainties are detailed in Fresenius Medical Care AG & Co. KGaA's reports filed with the U.S. Securities and Exchange Commission. Fresenius Medical Care AG & Co. KGaA does not undertake any responsibility to update the forward-looking statements in this release.
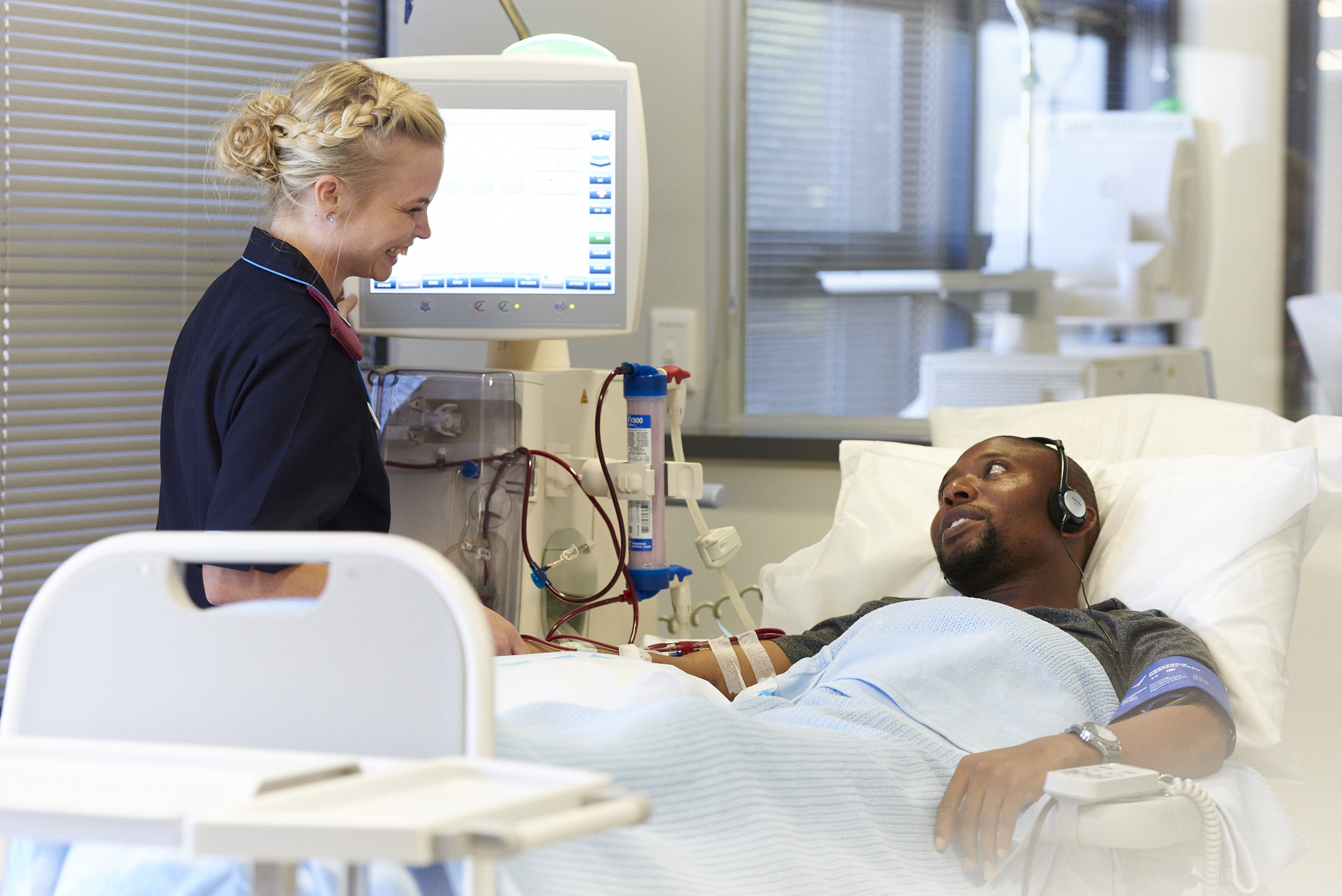
- The CONVINCE Trial study compares high-dose hemodiafiltration with standard, high-flux hemodialysis
- Study revealed a 23% decrease in mortality rates for patients treated with high-volume hemodiafiltration compared to those treated with more commonly used high-flux hemodialysis
- Exploration of methods to increase adoption of hemodiafiltration (HDF) making this therapeutic option accessible to patients
Fresenius Medical Care, the world's leading provider of products and services for individuals with renal diseases, participated in groundbreaking research that demonstrates that the mortality rate among kidney failure patients can be significantly reduced through the utilization of high-dose hemodiafiltration technology. Conducted by the CONVINCE consortium and led by the University Medical Center Utrecht, this international, randomized controlled trial marks a crucial milestone in comparing high-dose hemodiafiltration with standard, high-flux hemodialysis.
The study's findings reveal that patients treated with high-dose hemodiafiltration experienced a remarkable 23% decrease in mortality rates compared to those treated with the more commonly used high-flux hemodialysis. Hemodialysis represents the most prevalent form of dialysis for treating kidney failure. While techniques have improved over time, conventional high-flux hemodialysis primarily employs diffusion to remove small molecules and fluid from the blood. In contrast, high-dose hemodiafiltration incorporates both diffusion and convection techniques to eliminate larger molecules and effectively manage fluid replacement through convection.
Frank Maddux, MD, Chief Global Medical Officer of Fresenius Medical Care, expressed enthusiasm over the study's results, stating, "This meticulously designed and executed clinical study unquestionably demonstrates the efficacy of high volume hemodiafiltration for a significant portion of kidney failure patients. These findings have the potential to prompt significant changes in the standard treatment approach and, most importantly, contribute to reducing mortality rates among this vulnerable population in need of kidney replacement therapy."
Lead investigator Professor Peter Blankestijn from UMC Utrecht commented, "Our results unequivocally demonstrate the survival benefits of utilizing hemodiafiltration over hemodialysis in the treatment of kidney failure, akin to a remarkable 23% reduction in all-cause mortality. I am optimistic that hemodiafiltration can become the new standard of care.”
Chronic kidney disease poses a pressing global health challenge, affecting an estimated 830 million individuals worldwide, with nearly four million people reliant on dialysis. When the kidneys can no longer perform their vital functions, dialysis is employed to cleanse the blood by eliminating waste products and regulating fluid volume in the body.
The trial encompassed a total of 1,360 patients across 61 centers in eight European countries and followed up for a median of 30 months. Of these, 683 patients received high-dose hemodiafiltration and 677 received high-flux hemodialysis three times a week. The rate of mortality was 7.1 deaths per 100 patient-years in the group randomized to hemodiafiltration compared to 9.2 deaths per 100 patient-years in the hemodialysis group, corresponding to a relative reduction of 23%.
The CONVINCE trial was spearheaded by researchers from UMC Utrecht in collaboration with University College London (UCL), Charité Universitätsmedizin Berlin, University of Bari, The George Institute for Global Health, Imperial College London, and dialysis providers Fresenius Medical Care, Diaverum, and B. Braun Avitum.
While hemodialysis serves as the standard treatment in most countries, hemodiafiltration remains underutilized in certain regions and is yet to be adopted in places like the U.S. Fresenius Medical Care has taken the lead in developing machines that facilitate online fluid generation and innovative techniques for delivering hemodiafiltration.
"Fresenius Medical Care has a rich history of driving innovation in renal care, aimed at improving the lives of kidney disease patients worldwide," emphasized Frank Maddux, MD. "As pioneers in dialysis and membrane technologies, we will continue to explore methods that promote the adoption of hemodiafiltration, making this crucial therapeutic option more readily accessible to the patients we serve."
Disclaimer:
This release contains forward-looking statements that are subject to various risks and uncertainties. Actual results could differ materially from those described in these forward-looking statements due to various factors, including, but not limited to, changes in business, economic and competitive conditions, legal changes, regulatory approvals, impacts related to the COVID-19 pandemic results of clinical studies, foreign exchange rate fluctuations, uncertainties in litigation or investigative proceedings, and the availability of financing. These and other risks and uncertainties are detailed in Fresenius Medical Care AG & Co. KGaA's reports filed with the U.S. Securities and Exchange Commission. Fresenius Medical Care AG & Co. KGaA does not undertake any responsibility to update the forward-looking statements in this release.
__________
Notes to Editors:
Disclaimer: The information in this document is provided as is and no guarantee or warranty is given that the information is fit for any particular purpose. The user thereof uses the information at its sole risk and liability. The opinions expressed in the document are of the authors only and in no way reflect the European Commission’s opinions.
The CONVINCE study was exclusively supported by the European Commission Research & Innovation, Horizon 2020, Call H2020-SC1-2016-2017 under the topic SC1-PM-10-2017: Comparing the effectiveness of existing healthcare interventions in the adult population (grant no 754803).










