Fresenius today announced that further secondary endpoint data from a phase II/III study with removab® (catumaxomab) in patients with malignant ascites confirm clear benefits for patients treated with the antibody. Trial data show that removab significantly increases time to tumor progression and has a positive influence on overall survival time. Moreover, a prolonged interval between punctures was seen in the removab group compared to the control group and this effect was also observed beyond the end of study.
The results of the randomized study include treatment data from 258 patients with malignant ascites caused by various cancers. Most patients had late-stage disease with a median life expectancy of two to three months. The primary endpoint of the study had already demonstrated that patients receiving removab had a four-fold increased puncture-free survival over a therapy with puncture alone (median 46 vs. 11 days, p<0.0001). The median time to the first therapeutic puncture, a key secondary endpoint, improved to 77 days in the removab group versus 13 days in the control group (p<0.0001). In contrast to the primary endpoint, patients who died before the next puncture were not included in this metric.
There was a clear difference between the two study arms with regard to the time to progression (TTP) of the underlying cancer. The median TTP (secondary endpoint) for the 170 patients treated with removab was 111 days, compared to 35 days for the 88 patients of the control group (p<0.0001). In the subgroup of patients with ascites from ovarian cancer, removab-treated TTP was also 111 days, compared to patients in the control group with 35 days (p=0.0002). In addition, patients with other primary cancers receiving removab also showed significant improvement in TTP, with a median of 110 days versus 34 days with puncture-therapy alone (p<0.0001).
A positive trend was also observed for overall survival. Median overall survival in the 170 patients in the removab group was 72 days, compared to 68 days of the 88 patients in the control group (p=0.0846). In a prospectively planned evaluation of 131 patients treated per protocol, a median survival advantage of 18 days was shown (removab: 86 days vs. control group: 68 days, p=0.0085). removab treatment also showed a positive influence on the overall survival of ovarian cancer patients with a median survival of 110 days over 81 days for patients receiving puncture-therapy alone (p=0.1543). In patients with gastric cancer (the largest subgroup among patients with cancers other than ovarian cancer) the median survival advantage was 27 days (71 vs. 44 days, p=0.0313).
After the primary end point of the study (puncture-free survival) was reached, the data showed that puncture intervals in patients treated with removab continued to be longer than those of the patients in the control group. The intervals between the first and second puncture were 26 and 24 days in the ovarian and non-ovarian cancer group treated with removab vs. 13 and 16 days in the control group.
Secondary study endpoints and other data collected from patients after reaching the primary endpoint confirm the benefit of removab treatment for this patient group at a late stage of their disease. Collectively, the study results further indicate the efficacy of removab in the treatment of various primary cancers. "The results of the phase II/III study show clear benefits for patients being treated with removab", says Dr. Bernhard Ehmer, President Fresenius Biotech. "The results also suggest a direct antitumor effect of the trifunctional antibody. Primary and secondary endpoints as well as post study data are consistent, and demonstrate a pronounced positive trend. They all point in the right direction and this gives us confidence in our ongoing development program for ovarian and gastric cancer."
Fresenius Biotech confirms that the submission for marketing authorization with EMEA is expected in late 2007.
# # #
About the phase II/III study with removab
A total of 258 patients with end-stage cancer and recurrent symptomatic malignant ascites, who either no longer responded to or could no longer be treated with chemotherapy, were enrolled in this study. 129 of the patients had ovarian cancer and 129 participants had other forms of epithelial tumors (gastric 51 %, breast 10 %, pancreatic 7 %, colorectal 6 %, others 26 %). Participants in the study were randomized in a 2:1 ratio, with 170 patients randomized to treatment with removab and 88 patients to puncture therapy alone. After reaching the study endpoint 51% of patients in the control arm were also given removab (crossover). removab was administered intraperitoneally at ascending doses through infusions, following paracentesis on days 0, 3, 7, and 10. 131 patients received all four infusions of 10, 20, 50, and 150 µg.
Mode of action of trifunctional antibody removab (catumaxomab)
The therapeutic objective of trifunctional antibodies is to generate a stronger immune reaction against tumor cells. removab has two different antigen binding sites: While one arm of the antibody recognizes and binds to T-cells, the other arm binds EpCAM (epithelial cell adhesion molecule) that is overexpressed in many types of epithelial cancers. Immune effector cells with Fc receptors (macrophages, monocytes, dendritic cells and natural killer cells) can also bind the Fc region of intact trifunctional antibodies. This simultaneous binding subsequently results in the costimulation and activation of T-cells and accessory cells, enabling the generation of a strong immune response against tumor cells. Preclinical data also suggest a potential long-lasting effect to prevent cancer recurrence. Apart from removab two other trifunctional antibodies targeting other cancer antigens are currently undergoing clinical development.
Trifunctional Antibodies
Trifunctional antibodies are proteins that activate different cell types of the immune system simultaneously and target tumor cells specifically. Trifunctional antibodies therefore are very effective in destroying cancer cells and show a therapeutic effect even at very low doses. They are being developed by TRION Pharma GmbH.
# # #
Fresenius Biotech is a company within the Fresenius health care group and is focused on the development and marketing of biopharmaceuticals in the fields of oncology, immunology and regenerative medicine. For further information please visit www.fresenius-biotech.com.
Fresenius is a health care group with international operations, providing products and services for dialysis, hospital and outpatient medical care. In 2006, group sales were about 10.8 billion. On March 31, 2007 the Fresenius Group had 107.348 employees worldwide.
Trion Pharma is a biopharmaceutical company that develops and produces trifunctional antibodies based on a globally patented technology platform together with Fresenius Biotech in Munich. For further information please visit www.trionpharma.de.
# # #
Glossary
Puncture-free survival period: Period between the last infusion (control group: day of the puncture) and the first subsequent necessary puncture or death, which ever occurs first.
TTP (Time to Progression): Time to progression is the length of time between treatment and further growth of the primary tumor or metastases.
- Sales: € 5.59 billion, + 10 % at actual rates, + 15 % in constant currency
- EBIT: € 780 million, + 15 % at actual rates, + 20 % in constant currency
- Net income: € 195 million, + 39 % at actual rates, + 44 % in constant currency
- Excellent sales and earnings growth and further margin improvements in all business segments
Earnings outlook for 2007 raised
Based on the Group's strong financial results in the first half, Fresenius raises its earnings outlook for 2007. Net income is now expected to increase by ~25 % in constant currency. Previously, the Company expected net income to increase by 20 to 25 %. Group sales are expected to grow by 8 to 10 % in constant currency.
Sales – excellent organic growth
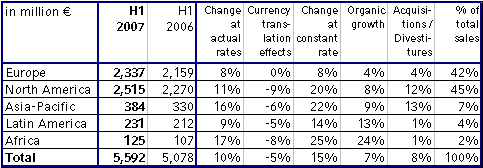
Group sales increased by 10 % to € 5,592 million (H1 2006: € 5,078 million). Organic sales growth was 7 %. Acquisitions contributed 10 %, in particular Renal Care Group, which was consolidated as from the second quarter of 2006. Divestitures reduced sales by 2 %. Currency translation effects had a negative impact of 5 %. This was mainly attributable to the average dollar rate depreciating 8 % against the euro in the first half of 2007 compared to previous year's period.
In North America sales grew significantly due to the Renal Care Group consolidation and an excellent organic growth rate of 8 %. In Europe sales increased by 8 % in constant currency, with organic growth of 4 %. Strong growth rates were achieved in the emerging markets with organic growth of 9 % in Asia-Pacific, 13 % in Latin America and 24 % in Africa.

Strong earnings growth
EBITDA increased by 18 % in constant currency and by 13 % at actual rates to € 977 million (H1 2006: € 867 million). Group operating income (EBIT) increased by 20 % in constant currency and by 15 % at actual rates to € 780 million (H1 2006: € 681 million; adjusted € 652 million including a gain from the divestiture of US dialysis clinics and one-time expenses related to the Renal Care Group acquisition). The strong earnings growth was driven by the successful operating results in all business segments. The Group's EBIT margin improved to 13.9 % (H1 2006: 13.4 %, adjusted margin: 12.8 %).
Group net interest was € -185 million (H1 2006: € -194 million, including one-time expenses of € 30 million for the early refinancing of Group debt).
The tax rate improved to 36.0 % (H1 2006: 42.3%; 37.4 % adjusted for the tax expense related to the divestiture of US dialysis clinics).
Minority interest increased to € 186 million (H1 2006: € 141 million), of which 93 % was attributable to the minority interest in Fresenius Medical Care.
Group net income also grew strongly by 44 % in constant currency and by 39 % at actual rates to € 195 million (H1 2006: € 140 million, including one-time expenses of € 16 million).
Earnings per ordinary share were € 1.26 and earnings per preference share were € 1.27 (H1 2006, adjusted for the February 2007 share split: ordinary share € 0.91, preference share € 0.92). This represents an increase of 38 % for both share classes.
High level of investment
Fresenius Group spent € 304 million for property, plant and equipment and intangible assets (H1 2006: € 225 million). Acquisition spending was € 221 million (H1 2006: € 3,408 million).
Strong cash flow
Operating cash flow increased by 48 % to € 553 million (H1 2006: € 373 million), mainly driven by the strong earnings increase. Cash flow before acquisitions and dividends increased by 60 % to € 256 million (H1 2006: € 160 million). Free cash flow after acquisitions (€ 162 million) and dividends (€ 188 million) was € -94 million (H1 2006: € -2,997 million).
Solid balance sheet structure
Total assets increased by 3 % in constant currency and by 2 % at actual rates to € 15,343 million (December 31, 2006: € 15,024 million). Current assets increased by 4 % to € 4,275 million (December 31, 2006: € 4,106 million). Non-current assets were € 11,068 million (December 31, 2006: € 10,918 million).
Shareholders' equity including minority interest grew by 3 % to € 5,895 million (December 31, 2006: € 5,728 million). The equity ratio (including minority interest) was 38.4 % (December 31, 2006: 38.1 %).
The Group's debt was € 5,909 million (December 31, 2006: € 5,872 million). The net debt/EBITDA ratio stood at 2.9 on June 30, 2007 (December 31, 2006: 3.0).
Employees
As of June 30, 2007, the Group had 108,860 employees (December 31, 2006: 104,872), an increase of 4 %.
Fresenius Biotech
Fresenius Biotech develops innovative therapies with trifunctional antibodies for the treatment of cancer as well as cell therapies for the treatment of the immune system. In the field of polyclonal antibodies, Fresenius Biotech has successfully marketed ATG-Fresenius S for many years. ATG-Fresenius S is an immunosuppressive agent used to prevent and treat graft rejection following organ transplantation.
In July 2007, Fresenius Biotech announced further secondary endpoint data from a phase II/III study with removab® (catumaxomab) in patients with malignant ascites. The data confirmed clear benefits for patients treated with the antibody. The trial data showed that removab significantly increases time to tumor progression and has a positive influence on overall survival time. Moreover, a prolonged time interval between therapeutic punctures for the treatment of malignant ascites was seen in the removab group compared to the control group. This effect was also observed beyond the end of the study. In December 2006 and March 2007, Fresenius Biotech already announced encouraging results on the primary study endpoint – puncture free survival.
The submission for marketing authorization with the European Medicines Agency (EMEA) for removab in malignant ascites is expected in late 2007.
The Phase II studies with the antibody rexomun® to treat breast cancer and with the antibody removab to treat gastric cancer are ongoing. These studies started in March 2006 and June 2006, respectively. A phase II study with removab for the treatment of patients with ovarian cancer was started in Europe.
In the first half of 2007, Fresenius Biotech's operating income (EBIT) was € -20 million. For 2007, Fresenius Biotech expects an EBIT of approximately € -50 million (2006: € -45 million).
The Business Segments
Fresenius Medical Care
Fresenius Medical Care is the world's leading provider of services and products for patients with chronic kidney failure. As of June 30, 2007, Fresenius Medical Care was serving 171,687 patients in 2,209 dialysis clinics.


- Continued strong organic sales development
- Excellent earnings growth
- 2007 outlook raised
Fresenius Medical Care achieved significant sales growth of 21 % to US$ 4,725 million (H1 2006: US$ 3,912 million). This was mainly driven by the strong organic growth of 8 % and by the consolidation of Renal Care Group (RCG) as from the second quarter of 2006. Sales in dialysis care increased by 22 % to US$ 3,556 million (H1 2006: US$ 2,924 million). In dialysis products, Fresenius Medical Care achieved sales of US$ 1,169 million (H1 2006: US$ 988 million), an increase of 18 %.
In North America Fresenius Medical Care's sales increased by 20 % to US$ 3,297 million (H1 2006: US$ 2,754). Sales outside North America ("International") grew by 23 % (in constant currency: 16 %) to US$ 1,428 million (H1 2006: US$ 1,158 million). This was driven by the positive operating performance in Europe, the Asia-Pacific region and in Latin America.
Fresenius Medical Care increased EBIT by 23 % to US$ 756 million (H1 2006: US$ 616 million, adjusted US$ 581 million including a gain from the divestiture of US dialysis clinics and one-time expenses related to the RCG acquisition). The EBIT margin was 16.0 % (H1 2006: 15.7 %, adjusted 14.8 %). Net income increased by 38 % to US$ 339 million (H1 2006: US$ 246 million, including one-time expenses of US$ 16 million).
Based on the strong operational performance in the first half of 2007, Fresenius Medical Care raises its outlook for the full year 2007 and now expects to achieve revenue of more than US$ 9.5 billion. This represents an increase of at least 12 %. Previously, Fresenius Medical Care expected revenue of approximately US$ 9.4 billion. Net income is now projected to be in the range of US$ 685 million to US$ 705 million in 2007. This represents an increase of between 19 % and 23 % on an adjusted basis as compared to 2006 after one-time effects. On a reported basis, this translates into an increase in net income of between 28 % and 31 %. Previously, Fresenius Medical Care expected net income in the range of US$ 675 million to US$ 695 million.
For further information, please see Fresenius Medical Care's press release at www.fmc-ag.com.
Fresenius Kabi
Fresenius Kabi offers infusion therapies and clinical nutrition for seriously and chronically ill patients in the hospital and out-patient environments. The company is also a leading provider of transfusion technology products.

- Excellent organic sales growth
- Continued strong EBIT margin increase in second quarter and first half of 2007
- 2007 outlook fully confirmed
In the first half of 2007, Fresenius Kabi increased sales by 5 % to € 986 million (H1 2006: € 937 million). Currency translation effects had an impact of -2 %. This was mainly due to the depreciation of currencies in South Africa, China and Canada. Organic growth was 7 %. In the second quarter of 2007, Fresenius Kabi achieved strong organic growth of 8 %.
In Europe (excluding Germany) organic sales growth was 5 % while sales in Germany were at previous year's level. In the second quarter of 2007, organic sales growth was 1 % in Germany. Outside Europe, Fresenius Kabi achieved organic sales growth of 22 % in the Asia-Pacific region in the first half of 2007. Organic sales growth in Latin America was 10 % and in other regions 11 %.
Fresenius Kabi continued its excellent earnings growth. In the first half of 2007, EBIT increased by 14 % to € 159 million (H1 2006: € 139 million). The EBIT margin improved to 16.1 % (H1 2006: 14.8 %). In the second quarter of 2007, Fresenius Kabi achieved an EBIT margin of 16.3 %. Net income increased by 45 % to € 87 million (H1 2006: € 60 million, including one-time expenses for early debt refinancing of € 11 million).
Fresenius Kabi fully confirms its outlook for the full year 2007. Organic sales growth is projected to be 6 to 8 %. Continued strong sales growth is anticipated from the regions outside Europe. Based on the positive sales projection and further manufacturing and logistics improvements Fresenius Kabi expects an EBIT margin of 16.0 to 16.5 % in 2007.
Fresenius ProServe
Fresenius ProServe is a leading German hospital operator with 58 facilities. Moreover, the company offers engineering and services for hospitals and other health care facilities.

- Continued strong sales and earnings growth
- Sale of Pharmatec to Robert Bosch GmbH completed June 30, 2007
- 2007 earnings outlook raised
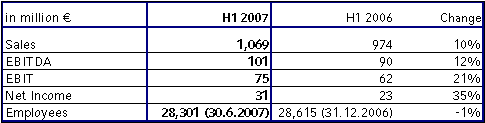
Fresenius ProServe's sales grew by 10 % to € 1,069 million (H1 2006: € 974 million). Organic sales growth was 2 %. EBIT increased by 21 % to € 75 million (H1 2006: € 62 million).
Sales in hospital operations (HELIOS Kliniken Group) grew by 16 % to € 890 million (H1 2006: € 767 million). The growth is mainly attributable to the acquisition of HUMAINE Kliniken, which was consolidated as from July 1, 2006. HELIOS achieved strong organic growth of 3 %. EBIT increased by 21 % to € 68 million, EBIT margin was 7.6 % (H1 2006: € 56 million and 7.3 %).
In the second quarter of 2007, HELIOS Kliniken inaugurated its newly constructed hospital Berlin-Buch. In parallel, HELIOS continued its growth strategy in the German hospital market. In July 2007, HELIOS agreed to acquire the Mariahilf hospital in Hamburg. The hospital has 255 beds and achieved revenues of € 26 million in 2006.
Sales in the engineering and services business was € 179 million (H1 2006: € 207 million). The decrease was mainly due to the sale of Pharmaplan, which was deconsolidated as of January 1, 2007. Organic growth was -2 %. EBIT was € 9 million (H1 2006: € 9 million).
Furthermore, Fresenius ProServe agreed to sell its subsidiary Pharmatec to Robert Bosch GmbH on May 1, 2007. In 2006, the company had sales of about € 30 million. The transaction was completed June 30, 2007.
Order intake in the engineering and services business was € 106 million (H1 2006: € 185 million). The decrease was due to the postponement of orders to the second half of 2007 and to a very strong order intake in the second quarter of 2006. Order backlog was € 379 million (December 31, 2006: € 428 million).
Based on the excellent financial results in the first half, Fresenius ProServe raises its 2007 EBIT outlook from previously € 160 to 170 million to now € ~170 million. The outlook for organic sales growth is confirmed at 2 to 3 %.
# # #
Conference call
As part of the publication of the results from the first half of 2007, a conference call will be held on August 2, 2007 at 2.00 p.m. CEDT (8.00 a.m. EDT). We invite all investors to follow the conference call over our web site under Investor Relations/Presentations. Following the conference, a recording of the call will be available.
Quarterly financial report
The report for the second quarter and the first half of 2007 will be available from August 14, 2007 under Investor Relations/Financial reports.
# # #
Fresenius is a health care group with international operations, providing products and services for dialysis, hospital and outpatient medical care. In 2006 group sales were approx. € 10.8 billion. On March 31, 2007 the Fresenius Group had 107,348 employees worldwide.
PDF-file includes:
Fresenius Group in Figures
- Consolidated statement of income (US GAAP) (unaudited)
- Key figures of the balance sheet (US GAAP) (unaudited)
- Cash flow statement (US GAAP) (unaudited)
- Segment reporting by business segment H1 2007 (US GAAP) (unaudited)
Having successfully completed its conversion into a European Company (SE) in July, Fresenius has now also given itself a new look.
The Group's corporate logo, colors and fonts have been revised and updated. The "F" in the new logo builds on the previous logo. Its form represents the Company's aspirations and continued growth. While the logo remained unchanged for over 13 years, Fresenius has grown significantly. Sales rose more than 10-fold from about € 1 billion in 1994 to € 10.8 billion in 2006, and the number of employees climbed from 8,000 in 1994 to about 109,000 this year.
Fresenius will continue to use its traditional blue color to represent the company, though in darker shade. The new corporate font "Interstate" is notable for its clarity. The business segments Fresenius Medical Care, Fresenius Kabi, Fresenius HELIOS and Fresenius VAMED will also review their corporate design.
You will find the new Fresenius company logo in print quality at http://www.fresenius.de/logos.
# # #
Fresenius is a health care group with international operations, providing products and services for dialysis, hospital and outpatient medical care. In 2006 group sales were approx. € 10.8 billion. On June 30, 2007 the Fresenius Group had 108,860 employees worldwide.
- Sales: € 8.4 billion, + 7 % at actual rates, + 11 % in constant currency
- EBIT: € 1.2 billion, + 12 % at actual rates, + 17 % in constant currency
- Net income: € 298 million, + 28 % at actual rates, + 32 % in constant currency
Outlook raised
Based on the Group's excellent financial results in the third quarter, Fresenius now expects net income to increase by more than 25 % in constant currency. Group sales are expected to grow by 9 to 10 % in constant currency. Previously, the Company expected net income to increase by ~25 % in constant currency and sales to grow by 8 to 10 % in constant currency.
Substantial growth: Group sales up 11 % in constant currency
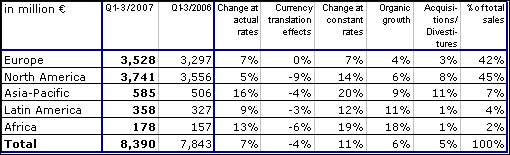
In the first three quarters Group sales increased by 11 % in constant currency and by 7 % at actual rates to € 8,390 million (Q1-3 2006: € 7,843 million). Organic sales growth was 6 %. Acquisitions contributed a further 6 %. Divestitures reduced sales growth by 1 %. Currency translation had a negative impact of 4 %.
In North America sales grew by 14 % in constant currency due to the Renal Care Group consolidation and an organic growth rate of 6 %. In Europe sales grew by 7 % in constant currency with organic sales growth contributing 4 %. Strong growth rates were achieved in the emerging markets with organic growth of 9 % in Asia-Pacific, 11 % in Latin America and 18 % in Africa.

Excellent earnings growth: net income up 32 % in constant currency
Group EBITDA increased by 15 % in constant currency and by 10 % at actual rates to € 1,485 million (Q1-3 2006: € 1,350 million). Group operating income (EBIT) grew by 17 % in constant currency and by 12 % at actual rates to € 1,184 million (Q1-3 2006: € 1,060 million; adjusted for the gain from the divestiture of US dialysis clinics and one-time expenses related to the Renal Care Group acquisition: € 1,036 million). The Group's EBIT margin improved to 14.1 % (Q1-3 2006: 13.5 %).
Group net interest was € -279 million (Q1-3 2006: € -295 million, including one-time expenses of € 30 million for the early refinancing of Group debt).
The tax rate was 36.0 % (Q1-3 2006: 40.9 %; adjusted for the tax expense related to the divestiture of US dialysis clinics: 37.8 %).
Minority interest increased to € 281 million (Q1-3 2006: € 219 million), of which 93 % was attributable to the minority interest in Fresenius Medical Care.
Group net income grew strongly by 32 % in constant currency and by 28 % at actual rates to € 298 million (Q1-3 2006: € 233 million, including one-time expenses of € 16 million).
Earnings per ordinary share were € 1.92 and earnings per preference share were € 1.93 (Q1-3 2006 adjusted for the February 2007 share split: ordinary share € 1.52, preference share € 1.53). This represents an increase of 26 % for both share classes.
Investments at a high level of € 727 million
Fresenius Group spent € 485 million for property, plant and equipment and intangible assets (Q1-3 2006: € 374 million). Acquisitions were € 242 million (Q1-3 2006: € 3,537 million).
Strong cash flow
Operating cash flow increased by 55 % to € 912 million (Q1-3 2006: € 588 million), driven by the strong earnings increase. In 2006, tax payments associated with the divestiture of US dialysis clinics had a negative effect. The cash flow margin was 10.9 % (Q1-3 2006: 7.5 %). Cash flow before acquisitions and dividends nearly doubled to € 447 million (Q1-3 2006: € 228 million). Free cash flow after acquisitions (€ 182 million) and dividends (€ 191 million) was € 74 million (Q1-3 2006: € -2,986 million).
Balance sheet structure: Net debt/EBITDA ratio improved
Fresenius Group's total assets increased by 4 % in constant currency and just slightly at actual rates to € 15,054 million (December 31, 2006: € 15,024 million). Current assets increased by 4 % to € 4,266 million (December 31, 2006: € 4,106 million). Non-current assets were € 10,788 million (December 31, 2006: € 10,918 million).
Shareholders' equity including minority interest grew by 4 % to € 5,946 million (December 31, 2006: € 5,728 million). The equity ratio (including minority interest) was 39.5 % (December 31, 2006: 38.1 %).
Group debt decreased by 5 % at actual rates and 1 % in constant currency to € 5,596 million (December 31, 2006: € 5,872 million). The net debt/EBITDA ratio improved to 2.7 as of September 30, 2007, well below the level of 3.0 as of December 31, 2006.
Number of employees increased
As of September 30, 2007, Fresenius increased the number of its employees by 5 % to 110,379 (December 31, 2006: 104,872).
Fresenius Biotech
Fresenius Biotech develops innovative therapies with trifunctional antibodies for the treatment of cancer as well as cell therapies for the treatment of the immune system. In the field of polyclonal antibodies, Fresenius Biotech has successfully marketed ATG-Fresenius S for many years. ATG-Fresenius S is an immunosuppressive agent used to prevent and treat graft rejection following organ transplantation.
After successful completion of the phase II/III study with removab® in patients with malignant ascites, submission for marketing authorization with the European Medicines Agency (EMEA) is expected for late 2007.
For the future marketing of removab in the USA and Japan, Fresenius Biotech is in discussions with potential partners.
Fresenius Biotech and Nabi Biopharmaceuticals agreed to terminate the agreement for the clinical development and marketing of ATG-Fresenius S in North America. Fresenius Biotech will assume responsibility for the clinical development and registration of ATG-Fresenius S in the US and continue with the ongoing phase III study.
In the first three quarters of 2007, Fresenius Biotech's operating income (EBIT) was € -33 million. For the full year 2007, Fresenius Biotech continues to expect EBIT of approximately € -50 million (2006: € -45 million).
The Business Segments
Fresenius Medical Care
Fresenius Medical Care is the world's leading provider of services and products for patients with chronic kidney failure. As of September 30, 2007, Fresenius Medical Care was serving 172,227 patients in 2,221 dialysis clinics.

- Strong organic sales growth of 7 %
- EBIT margin improved
- Earnings outlook at upper end expected
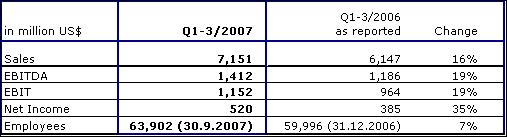
Fresenius Medical Care achieved strong sales growth of 16 % to US$ 7,151 million (Q1-3 2006: US$ 6,147 million). This was mainly driven by the strong organic growth of 7 % and by the consolidation of Renal Care Group (RCG). Sales in dialysis care increased by 16 % to US$ 5,357 million (Q1-3 2006: US$ 4,628 million). In dialysis products, sales grew by 18 % to US$ 1,794 million (Q1-3 2006: US$ 1,519 million).
In North America, sales growth was 14 % to US$ 4,957 million (Q1-3 2006: US$ 4,367 million). Sales outside North America ("International" segment) grew by 23 % (in constant currency: 15 %) to US$ 2,194 million (Q1-3 2006: US$ 1,780 million). This was driven by positive operating performance in Europe, the Asia-Pacific region and in Latin America.
EBIT rose by 19 % to US$ 1,152 million (Q1-3 2006: US$ 964 million; adjusted for the gain from the divestiture of US dialysis clinics and one-time expenses related to the RCG acquisition: US$ 936 million). The EBIT margin was 16.1 % (Q1-3 2006: 15.7 %; adjusted 15.2 %). Net income increased by 35 % to US$ 520 million (Q1-3 2006: US$ 385 million, including one-time expenses of US$ 20 million).
Fresenius Medical Care confirms its outlook for the full year 2007 and expects to achieve revenue of more than US$ 9.5 billion. This represents an increase of at least 12 %. Net income was projected to be in the range of US$ 685 million to US$ 705 million in 2007. Based on the strong performance in the third quarter, Fresenius Medical Care now expects the net income to be at the upper end of this guidance.
For further information, please see Fresenius Medical Care's press release at www.fmc-ag.com.
Fresenius Kabi
Fresenius Kabi offers infusion therapies and clinical nutrition for seriously and chronically ill patients in the hospital and out-patient environments. The company is also a leading provider of transfusion technology products.

- Strong organic sales growth of 7 %
- EBIT margin improves by 100 basis points to 16.2 %
- 2007 outlook fully confirmed
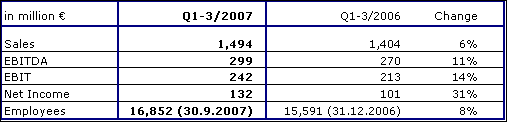
Fresenius Kabi increased sales by 6 % to € 1,494 million (Q1-3 2006: € 1,404 million). Currency translation effects had a negative impact of 2 %. This was mainly due to the depreciation of currencies in South Africa, China and Canada. Organic growth was 7 %. In the third quarter of 2007, Fresenius Kabi achieved an excellent organic growth of 8 %.
In Europe (excluding Germany) organic sales growth was 5 %. In Germany organic sales growth was 1 %. In the Asia-Pacific region Fresenius Kabi achieved significant organic sales growth of 22 %. Organic sales growth in Latin America was 10 % and in other regions 10 %.
Fresenius Kabi continued its excellent earnings growth. EBIT grew by 14 % to € 242 million (Q1-3 2006: € 213 million). The EBIT margin improved to 16.2 % (Q1-3 2006: 15.2 %). Fresenius Kabi reported strong growth in net income of 31 % to € 132 million (Q1-3 2006: € 101 million, including one-time expenses for early debt refinancing of € 11 million).
Fresenius Kabi fully confirms its outlook for the full year 2007. Organic sales growth is projected to be well into the 6 to 8 % range. Continued very strong sales growth is anticipated in particular from the regions outside Europe. Based on the positive sales projection and further manufacturing and logistics improvements Fresenius Kabi expects an EBIT margin of 16.0 to 16.5 % in 2007.
Fresenius ProServe
Fresenius ProServe is a leading German hospital operator with 58 facilities. Moreover, the company offers engineering and services for hospitals and other health care facilities.
- HELIOS achieves further operating margin improvement
- VAMED wins contract worth more than € 100 million
- Sales guidance for 2007 fully confirmed, EBIT guidance increased
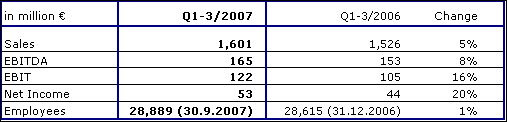
Fresenius ProServe achieved sales growth of 5 % to € 1,601 million (Q1-3 2006: € 1,526 million). Organic growth was 1 %, held back by delayed project revenues at VAMED. Acquisitions contributed 9 % whereas divestitures, primarily Pharmaplan and Pharmatec, had a negative impact of 5 %.
EBIT increased by 16 % to € 122 million (Q1-3 2006: € 105 million).
Sales in hospital operations (HELIOS Kliniken Group) increased by 12 % to € 1,348 million (Q1-3 2006: € 1,204 million). HELIOS achieved strong organic growth of 3 %. EBIT increased by 18 % to € 111 million (Q1-3 2006: € 94 million). The EBIT margin improved by 40 basis points to 8.2 %. In the third quarter of 2007, HELIOS reached an excellent EBIT margin of 9.4 % (Q3 2006: 8.7 %).
In July 2007, HELIOS agreed to acquire the Mariahilf hospital in Hamburg. The completion of the transaction is currently delayed by a legal proceeding against the seller of the hospital.
Sales in the engineering and services business were € 253 million (Q1-3 2006: € 322 million). The decrease is mainly due to the divestitures of Pharmaplan and Pharmatec, which were deconsolidated as of January 1, 2007, and June 30, 2007, respectively. Organic growth in the first three quarters was -7 % due to project delays at VAMED. EBIT was € 13 million (Q1-3 2006: € 14 million).
Order intake in the engineering business was € 245 million (Q1-3 2006: € 291 million). The decrease was due to the deconsolidation of the above-mentioned companies and the postponement of orders. In the third quarter of 2007, order intake increased by 31 % compared to the prior-year figure. This was mainly attributable to a contract worth more than € 100 million. The project includes the construction of a health and spa resort in Vienna/Austria. VAMED continues to expect an increase in its order intake in 2007 over 2006.
Due to its substantial order backlog of € 476 million (December 31, 2006: € 428 million) and its current view on the fourth quarter, VAMED remains confident to increase its sales in 2007 over 2006.
Based on the excellent financial results in 2007 to date, Fresenius ProServe raises its 2007 EBIT outlook from previously € ~170 million to more than € 170 million. The outlook for organic sales growth is confirmed at 2 to 3 %.
Press Conference
As part of the publication of the results for the first three quarters of 2007, a press conference will be held at the Fresenius headquarters in Bad Homburg on October 31, 2007 at 10 a.m. CET. All journalists are cordially invited to follow the conference in a live broadcast over out website at Press / Audio/Video Service. Following the meeting, a recording of the conference will be available as video-on-demand.
# # #
Fresenius is a health care group with international operations, providing products and services for dialysis, hospital and outpatient medical care. In 2006 group sales were approx. € 10.8 billion. On September 30, 2007 the Fresenius Group had 110,379 employees worldwide.
PDF-file includes:
Fresenius Group in Figures
- Consolidated statement of income (US GAAP) (unaudited)
- Key figures of the balance sheet (US GAAP) (unaudited)
- Cash flow statement (US GAAP) (unaudited)
- Segment reporting by business segment Q1-3 2007 (US GAAP) (unaudited)
Fresenius Biotech has dispatched the marketing authorization application for the trifunctional antibody Removab (INN: catumaxomab) in patients with malignant ascites to the European Medicines Agency (EMEA) as planned. The company applies for the EU authorization of Removab for the intraperitoneal treatment of malignant ascites in patients with epithelial cancers where no standard therapy is available or no longer feasible. The results of the phase II/III pivotal study announced in December 2006 as well as in March and July 2007 are an essential part of the marketing authorization application. In addition to the clinical results, the application contains preclinical data as well as production and product quality information. The scientific assessment of the marketing authorization application will start in early 2008 after the completion of the validation by EMEA.
Dr. Ulf M. Schneider, Chairman of the Management Board of Fresenius SE, commented: "The marketing authorization application for our Removab antibody is an important milestone for Fresenius Biotech. The results of the phase II/III pivotal study show clear benefits for patients treated with Removab. We believe that Removab could become a new therapy option for malignant ascites. We are encouraged to continue our Removab clinical trial program and will focus on applications in solid tumors."
Trifunctional Antibodies
Trifunctional antibodies are proteins that activate different cell types of the immune system simultaneously and direct these to the tumor cells by a targeted approach. Trifunctional antibodies therefore are very effective in destroying cancer cells and show a therapeutic effect even at very low doses. They are being developed by TRION Pharma GmbH.
Mode of action of trifunctional antibody removab (catumaxomab)
The therapeutic objective of trifunctional antibodies is to generate a stronger immune reaction against tumor cells. removab has two different antigen binding sites: While one arm of the antibody recognizes and binds to T-cells, the other arm binds EpCAM (epithelial cell adhesion molecule) that is overexpressed in many types of epithelial cancers. Immune effector cells with Fc receptors (macrophages, monocytes, dendritic cells and natural killer cells) can also bind the Fc region of intact trifunctional antibodies. This simultaneous binding subsequently results in the costimulation and activation of T-cells and accessory cells, enabling the generation of a strong immune response against tumor cells. Preclinical data also suggest a potential long-lasting effect to prevent cancer recurrence. Apart from removab two other trifunctional antibodies targeting other cancer antigens are currently undergoing clinical development.
Fresenius Biotech is a company within the Fresenius health care group and is focused on the development and marketing of biopharmaceuticals in the fields of oncology, immunology and regenerative medicine. For further information please visit www.fresenius-biotech.com.
Fresenius is a health care group with international operations, providing products and services for dialysis, hospital and outpatient medical care. In 2006, group sales were approx. € 10.8 billion. On September 30, 2007 the Fresenius Group had 110,379 employees worldwide.
TRION Pharma is a biopharmaceutical company that develops and produces trifunctional antibodies based on a globally patented technology platform together with Fresenius Biotech in Munich. For further information please visit www.trionpharma.de.
Glossary
Epithelial tumors: tumors that result from degenerated cells of epithelial origin.
This release contains forward-looking statements that are subject to various risks and uncertainties. Actual results could differ materially from those described in these forward-looking statements due to certain factors, including changes in business, economic and competitive conditions, regulatory reforms, foreign exchange rate fluctuations, uncertainties in litigation or investigative proceedings, and the availability of financing. Fresenius does not undertake any responsibility to update the forward-looking statements in this release.
Board of Management: Dr. Ulf M. Schneider (President and CEO), Rainer Baule, Andreas Gaddum, Dr. Jürgen Götz, Dr. Ben Lipps, Stephan Sturm
Supervisory Board: Dr. Gerd Krick (Chairman)
Registered Office: Bad Homburg, Germany/Commercial Register No. HRB 10660
Fresenius AG today provided details regarding its contemplated bond issue announced in October 2005. The Company intends to issue € 1.0 billion in Senior Notes (the "Senior Notes") through its wholly-owned subsidiary Fresenius Finance B.V. (the "Issuer"), subject to market conditions. The net proceeds from the Senior Notes offering (the "Senior Notes Offering"), along with the proceeds from our recent capital increase, finance the acquisition of HELIOS Kliniken GmbH and the proposed repurchase of the Issuer's outstanding € 300 million 7.750 % Series A Senior Notes due 2009, callable in 2006, by way of a cash tender (the "Tender Offer") and will be used for general corporate purposes.
Fresenius AG expects to complete the Senior Notes Offering before the end of January 2006.
The proposed Senior Notes Offering will not be registered under the Securities Act of 1933, but will be offered in the United States pursuant to an exemption from registration under Rule 144A, as well as outside the United States under Regulation S.
Credit Suisse First Boston (Europe) Limited and Morgan Stanley & Co. International Limited have been mandated as Joint Book-Running Lead Managers and Dresdner Bank AG London Branch as Joint Lead Manager of the proposed Senior Notes Offering.
The Tender Offer is subject to the terms described in the tender offer memorandum dated January 3, 2006.
Fresenius is a health care group with international operations, providing products and services for dialysis, hospital and the ambulatory medical care of patients. In 2004, sales were € 7.27 billion. On December 31, 2004 the Fresenius Group had 68,494 employees worldwide.
This announcement is for distribution only to persons who (i) have professional experience in matters relating to investments falling within Article 19(5) of the Financial Services and Markets Act 2000 (Financial Promotion) Order 2005 (as amended, the "Financial Promotion Order"), (ii) are persons falling within Article 43 of the Financial Promotion Order (iii) are persons falling within Article 49(2)(a) to (d) ("high net worth companies, unincorporated associations etc") of the Financial Promotion Order, (iv) are outside the United Kingdom, or (v) are persons to whom this announcement can otherwise be lawfully communicated or caused to be communicated (all such persons together being referred to as "relevant persons"). This announcement is directed only at relevant persons and must not be acted on or relied on by persons who are not relevant persons. Any investment or investment activity to which this announcement relates is available only to relevant persons and will be engaged in only with relevant persons.
This announcement is not an offer for sale of securities in the United States. Securities may not be sold in the United States absent registration or an exemption from registration under the U.S. Securities Act of 1933, as amended (re "Securities Act"). The Senior Notes referred to herein have not been and will not be registered under the Securities Act and Fresenius does not intend to register any portion of the offering in the United States or to conduct a public offering of securities in the United States. Copies of this announcement are not being made and may not be distributed or sent into the United States, Canada, Japan or Australia. It may be unlawful to distribute this announcement in certain other jurisdictions. The information in this announcement does not constitute an offer of securities for sale in Canada, Japan or Australia.
This announcement or any related documents are not being distributed in the context of a public offer in France within the meaning of Article L. 411-1 of the French Monetary and Financial Code (Code monétaire et financier), and thus neither this announcement nor any prospectus has been or will be submitted to the Autorité des Marchés Financiers for approval in France and accordingly may not and will not be distributed to the public in France. The offer of the Senior Notes is not being made and will not be made to the public in France except to (i) qualified investors (investisseurs qualifiés) and/or a restricted group of investors (cercle restreint d'investisseurs), in each case, acting for their own account, all as defined in, and in accordance with, Articles L. 411-1, L. 411-2, D. 411-1 and D. 411-2 of the French Monetary and Financial Code and/or (ii) persons providing portfolio management investment services acting for third parties.
This announcement does not constitute an offer to sell notes or a solicitation to buy notes in Germany. There will be no public offering of notes in Germany. Any offering of notes can only be made in accordance with the German Securities Prospectus Act (Wertpapierprospektgesetz, WpPG). No selling document pursuant to the German Securities Prospectus Act has been or will be published in relation to the Senior Notes. Therefore, this announcement and any other offering material in relation to the Senior Notes is directed only at persons who are "qualified investors" within the meaning of sec. 2 No. 6 of the German Securities Prospectus Act.
This announcement is an advertisement and is not a prospectus for the purposes of EU Directive 2003/71/EC (the "Directive"). This announcement and the offering of Senior Notes when made are only addressed to and directed at persons in member states of the European Economic Area who are "qualified investors" within the meaning of Article 2(1)(e) of the Directive.
Fresenius Biotech today announced that Enzon Pharmaceuticals, Inc. has decided not to continue the clinical development of the immunosuppressant ATG-Fresenius S for the U.S. market. Enzon terminated the agreement as a consequence of ongoing efforts to redirect its research and development investments to projects strategically aligned with its current business objectives, including an increasing focus on cancer and adjacent therapeutic areas. Fresenius is currently in negotiations with potential partners to continue the ongoing clinical development. During a transition period, Enzon will continue to fulfill its clinical and regulatory obligations.
ATG-Fresenius S is an immunosuppressive agent made from a polyclonal antibody and is used to suppress organ rejection following transplantation. Fresenius currently markets the drug in more than 60 countries and it generated about € 17 million in sales in 2004.
Fresenius Biotech GmbH is a company of the Fresenius Health Care Group, focused on the development and marketing of biopharmaceuticals in the fields of oncology, immunology and regenerative medicine.
This release contains forward-looking statements that are subject to certain risks and uncertainties. Future results could differ materially from those described in these forward-looking statements due to various factors, e.g., changes in the business, economic and competitive environment, regulatory reforms, results of clinical trials, foreign exchange rate fluctuations, uncertainties in litigation or investigative proceedings, and the availability of financing. Fresenius does not undertake any responsibility to update the forward-looking statements in this release.
Fresenius AG today announced that it has successfully priced its €1 billion offering of Senior Notes issued by its wholly-owned subsidiary Fresenius Finance B.V. (the "Issuer").
The issue includes two tranches:
- €500 million principal amount, at an issue price of 99.541 %, bearing interest at 5.0 % for the Senior Notes due 2013, non-callable,
- €500 million principal amount, at an issue price of 99.314 %, bearing interest at 5.5 % for the Senior Notes due 2016, callable from 2011 by the Issuer.
The net proceeds from the Senior Notes offering, along with the proceeds from the Company's recent capital increase, will finance the acquisition of HELIOS Kliniken GmbH and the repurchase of a portion of the Issuer's 7.75 % Series A Senior Notes and will also be used for general corporate purposes. The offering of Senior Notes will be closed and funded on January 20, 2006.
The transaction was well received by investors, being substantially oversubscribed.
Credit Suisse First Boston (Europe) Limited and Morgan Stanley & Co. International Limited acted as Joint Book-Running Lead Managers and Dresdner Kleinwort Wasserstein acted as Joint Lead Manager of the Senior Notes offering.
In conjunction with the above, Fresenius AG priced the offer to repurchase its €300 million aggregate principal amount of the Issuer's outstanding 7.75 % Series A Senior notes due 2009, listed on the Luxembourg Stock Exchange. Fresenius repurchases €212,079,000 aggregate principal amount of Notes, representing a participation rate for the tender offer of approx. 71 %. The repurchase price is 105.168 %, or €1,051.68 per €1,000 nominal value of Notes, plus accrued interest from the last interest payment date.
Fresenius is a health care group with international operations, providing products and services for dialysis, hospital and the ambulatory medical care of patients. In 2004, sales were € 7.27 billion. On December 31, 2004 the Fresenius Group had 68,494 employees worldwide.
This announcement is for distribution only to persons who (i) have professional experience in matters relating to investments falling within Article 19(5) of the Financial Services and Markets Act 2000 (Financial Promotion) Order 2005 (as amended, the "Financial Promotion Order"), (ii) are persons falling within Article 43 of the Financial Promotion Order (iii) are persons falling within Article 49(2)(a) to (d) ("high net worth companies, unincorporated associations etc") of the Financial Promotion Order, (iv) are outside the United Kingdom, or (v) are persons to whom this announcement can otherwise be lawfully communicated or caused to be communicated (all such persons together being referred to as "relevant persons"). This announcement is directed only at relevant persons and must not be acted on or relied on by persons who are not relevant persons. Any investment or investment activity to which this announcement relates is available only to relevant persons and will be engaged in only with relevant persons.
This announcement is not an offer for sale of securities in the United States. Securities may not be sold in the United States absent registration or an exemption from registration under the U.S. Securities Act of 1933, as amended (re "Securities Act"). The Senior Notes referred to herein have not been and will not be registered under the Securities Act and Fresenius does not intend to register any portion of the offering in the United States or to conduct a public offering of securities in the United States. Copies of this announcement are not being made and may not be distributed or sent into the United States, Canada, Japan or Australia. It may be unlawful to distribute this announcement in certain other jurisdictions. The information in this announcement does not constitute an offer of securities for sale in Canada, Japan or Australia.
This announcement or any related documents are not being distributed in the context of a public offer in France within the meaning of Article L. 411-1 of the French Monetary and Financial Code (Code monétaire et financier), and thus neither this announcement nor any prospectus has been or will be submitted to the Autorité des Marchés Financiers for approval in France and accordingly may not and will not be distributed to the public in France. The offer of the Senior Notes is not being made and will not be made to the public in France except to (i) qualified investors (investisseurs qualifiés) and/or a restricted group of investors (cercle restreint d'investisseurs), in each case, acting for their own account, all as defined in, and in accordance with, Articles L. 411-1, L. 411-2, D. 411-1 and D. 411-2 of the French Monetary and Financial Code and/or (ii) persons providing portfolio management investment services acting for third parties.
This announcement does not constitute an offer to sell notes or a solicitation to buy notes in Germany. There will be no public offering of notes in Germany. Any offering of notes can only be made in accordance with the German Securities Prospectus Act (Wertpapierprospektgesetz, WpPG). No selling document pursuant to the German Securities Prospectus Act has been or will be published in relation to the Senior Notes. Therefore, this announcement and any other offering material in relation to the Senior Notes is directed only at persons who are "qualified investors" within the meaning of sec. 2 No. 6 of the German Securities Prospectus Act.
This announcement is an advertisement and is not a prospectus for the purposes of EU Directive 2003/71/EC (the "Directive"). This announcement and the offering of Senior Notes when made are only addressed to and directed at persons in member states of the European Economic Area who are "qualified investors" within the meaning of Article 2(1)(e) of the Directive.
Fresenius Medical Care AG ("the Company") (Frankfurt Stock Exchange: FME, FME3) (NYSE: FMS, FMS-p), the world's largest provider of Dialysis Products and Services, today announced the final results of the Company's preference share conversion offer. During the four-week conversion period which ended February 3, 2006, 26,629,422 preference shares were tendered for conversion. This represents approximately 96% of all outstanding preference shares. This number includes 699,949 preference shares represented by American Depositary Shares (ADS), which represents approximately 92% of all the outstanding preference ADS as of February 3, 2006. Three preference ADSs represent one preference share.
In connection with the conversion, preference shareholders had to pay a conversion premium of €9,75 per tendered preference share (€3.25 per preference ADS) to the Company. Upon effectiveness of the conversion Fresenius Medical Care will receive total gross proceeds of approximately
€ 260 million.
The transformation of the legal form of the Company from an Aktien-gesellschaft into a partnership limited by shares (Kommanditgesellschaft auf Aktien – KGaA) is scheduled for the evening of Friday, February 10, 2006. The transformation will become effective upon registration with the commercial register of the local court (Amtsgericht), in Hof an der Saale (Germany).
Monday, February 13, 2006, is scheduled to be the first trading day of the Fresenius Medical Care AG & Co. KGaA shares including the new ordinary shares, on German stock exchanges and the first trading day of Fresenius Medical Care AG & Co. KGaA ADSs on the New York Stock Exchange.
Anticipated Time Schedule:
February 10, 2006
Scheduled trading stop of Fresenius Medical Care AG preference and ordinary shares and preference shares tendered for conversion will continue from 05:30 p.m. Central European Time onwards. Thereafter, the transformation into KGaA and conversion become effective.
February 13, 2006
First trading day of the shares of Fresenius Medical
Care AG & Co. KGaA; First trading day of American Depositary Shares (ADS) of Fresenius Medical Care AG & Co. KGaA on the New York Stock Exchange.
At the earliest:
February 13, 2006
Delivery of new ordinary shares resulting from conversion.
# # #
Fresenius Medical Care AG is the world's largest, integrated provider of products and services for individuals undergoing dialysis because of chronic kidney failure, a condition that affects more than 1,300,000 individuals worldwide. Through its network of approximately 1,670 dialysis clinics in North America, Europe, Latin America, Asia-Pacific and Africa, Fresenius Medical Care provides dialysis treatment to approximately 130,400 patients around the globe. Fresenius Medical Care is also the world's leading provider of dialysis products such as hemodialysis machines, dialyzers and related disposable products.
For more information about Fresenius Medical Care visit the Company's website at www.fmc-ag.com.
- Sales: € 7.9 billion, + 8 % at actual rates and in constant currency
- EBIT: € 969 million, + 15 % at actual rates , + 14 % in constant currency
- Net incombe: € 222 million, + 32 % at actual rates, + 31 % in constant currency
- Strong sales and earnings growth at Fresenius Medical Care
- Excellent business performance and EBIT margin increase to 13.9 % at Fresenius Kabi
- Fresenius ProServe within expectations; order intake in project business +40 %
- Strong sales and earnings growth expected for 2006
Dividend increase proposed
2005 was a very successful year for Fresenius. Based on the Group's excellent financial results, for the 13th consecutive year the Management Board will propose to the Supervisory Board a dividend increase to € 1.48 per ordinary share (2004: € 1.35) and € 1.51 per preference share (2004: € 1.38). As the 9.4 million new shares from the capital increase in December 2005 are fully entitled to the 2005 dividend, the total dividend distribution will be € 75.8 million (2004: € 55.9 million).
Positive Group outlook for 2006
For 2006, Fresenius expects to achieve sales growth of about 30 % to approximately € 10.5 billion including Renal Care Group and an organic growth of 5-6 %.
Net income is projected to grow by more than 30 % in constant currency. The net income guidance already includes an amount of approx. € 30 million (after tax) associated with expected one-time expenses for the integration of Renal Care Group and the refinancing of debt as well as for costs related to the change of accounting principles for stock options. Due to the higher number of shares issued in December 2005, earnings per share are projected to increase by approximately 10 % in constant currency.
Investments in property, plant and equipment and intangible assets are projected to increase to approximately € 550 - 600 million.
Strong organic sales growth
In 2005, Group sales increased 8 % to € 7,889 million (2004: € 7,271 million). Organic growth contributed 7 % and acquisitions 2 %. Divestments had a -1 % effect on sales. Currency translation had hardly any impact.
Remarkable sales growth of 8 % was achieved each in our main markets North America and Europe. Latin America with sales growth of 30 % and Africa with 16 % performed strongly. In Asia-Pacific, Fresenius Medical Care and particularly Fresenius Kabi achieved an excellent sales increase. Sales of Fresenius ProServe, however, decreased due to the lower project volume in this region.

Sales contribution of the three business segments:

Excellent earnings growth
EBITDA increased 11 % to € 1,289 million (2004: € 1,160 million). Group EBIT rose 15 % at actual rates and 14 % in constant currency to € 969 million (2004: € 845 million). The Group EBIT margin improved to 12.3 % (2004: 11.6 %).
Group net interest improved to € -203 million (2004: € -209 million) primarily as a result of a lower debt level in combination with lower interest rates from various refinancing measures.
The tax rate for 2005 was 38.9 % (2004: 39.8 %).
Minority interest was € 246 million (2004: € 215 million). 96 % was attributable to the minority interest of Fresenius Medical Care.
Group net income grew significantly by 32 % at actual rates and by 31 % in constant currency to € 222 million (2004: € 168 million). Key growth drivers were the excellent operating results of Fresenius Medical Care and Fresenius Kabi as well as reduced financing costs and a lower tax rate.
Earnings per ordinary share rose to € 5.28 (2004: € 4.08) while earnings per preference share rose to € 5.31 (2004: € 4.11). This is an increase of 29 % for both share classes. The average number of shares grew to 41.88 million primarily due to the capital increase.
Investments at record level
Total Group investments increased to € 2.25 billion (2004: € 421 million). € 1.89 billion was spent on acquisitions (2004: € 113 million), including € 1.5 billion for HELIOS. € 353 million was spent for property, plant and equipment and intangible assets (2004: € 308 million).
Solid cash flow performance
Fresenius achieved a good operating cash flow of € 780 million (2004: € 851 million). Key drivers were the significant improvement in earnings whereas income tax payments of Fresenius Medical Care for prior years had a negative effect. Cash flow before acquisitions and dividends was € 449 million (2004: € 565 million). Dividends of € 132 million and about 20 % of the 2005 acquisitions (net) were financed through cash flow. The balance was financed through bank debt and the capital increase.
Solid balance sheet structure
Total assets increased 42 % to € 11,594 million (December 31, 2004: € 8,188 million). In constant currency, total assets grew 33 %. The substantial increase in assets is acquisition-related, mainly due to the HELIOS acquisition. Current assets increased 28 % to € 3,531 million (December 31, 2004: € 2,755 million). In constant currency, current assets grew 21 %. Non-current assets were € 8,063 million (2004: € 5,433 million), a constant currency increase of 39 %. This was primarily due to an increase in goodwill.
Group debt increased 28 % to € 3,502 million (December 31, 2004: € 2,735 million) due to acquisition financing. In constant currency, the increase was 24 %.
Including HELIOS's EBITDA contribution the net debt/EBITDA ratio was 2.3 (December 31, 2004: 2.2).
Shareholders' equity including minority interest was € 5,130 million, a 40 % constant-currency increase (December 31, 2004: € 3,347 million). This was due to the excellent earnings development and the proceeds from the capital increase. The equity ratio including minority interest improved to 44.2 % (December 31, 2004: 40.9 %).
Employee numbers continue to grow
As of December 31, 2005, the Group had 91,971 employees worldwide (December 31, 2004: 68,494). The increase of 23,477 employees is principally due to the acquisition of HELIOS.
Fresenius Biotech
Fresenius Biotech develops innovative therapies with trifunctional antibodies for the treatment of cancer as well as cell therapies for the treatment of the immune system. In the field of polyclonal antibodies, Fresenius Biotech has successfully marketed ATG-Fresenius S for many years. ATG-Fresenius S is an immunosuppressive agent used to prevent and treat graft rejection following organ transplantation.
In the field of the trifunctional antibody therapies for the treatment of cancer, Fresenius Biotech expects results from the clinical study for ovarian cancer in the first half of 2006. The results from the malignant ascites and malignant pleural effusion studies are expected in the second half of 2006. Following the positive results from two phase I studies for the treatment of peritoneal carcinomatosis and breast cancer, phase II studies for the treatment of gastric cancer and breast cancer are being prepared.
In 2005, Fresenius Biotech's EBIT was € -40.6 million (2004: € -28 million). This development was within our expectations and is a result of the increased research and development spending. For 2006, Fresenius Biotech‘s EBIT is expected to be in the range of € -45 to -50 million, largely due to the expanded clinical study program.
The Business Segments
Fresenius Medical Care
Fresenius Medical Care is the world's leading provider of products and services for patients with chronic kidney failure. As of December 31, 2005, Fresenius Medical Care was serving approximately 131,450 patients (+6 %) in 1,680 dialysis clinics (+4 %). The company delivered about 19.7 million treatments in 2005 (+5 %).

- Strong sales and earnings growth continued
- Transformation into KGaA and conversion of preference shares successfully completed
- Closing of the Renal Care Group (RCG) acquisition expected in 1st quarter of 2006
EBIT rose 10 % to US$ 939 million (2004: US$ 852 million) and the EBIT margin was 13.9 % (2004: 13.7 %). This figure includes one-time costs of US$ 22 million associated with the transformation of Fresenius Medical Care's legal form into a KGaA and related legal fees and costs concerning the settlement of shareholder litigation. Net income including one-time costs grew by 13 % to US$ 455 million in 2005 (2004: US$ 402 million).
For the full year 2006, Fresenius Medical Care expects revenue growth at constant currencies of approximately 25 % on a pro forma basis, giving effect to the RCG merger as compared to 2005 reported revenues. Pro forma amounts assume consolidation of RCG's operations into Fresenius Medical Care for the full twelve months of 2006. For the full year 2006, Fresenius Medical Care expects to report revenue of more than US$ 8 billion.
Fresenius Medical Care's projected net income growth on a pro forma basis for 2006 is expected to be between 10 and 15 %, based on the US$ 472 million net income excluding one-time costs, achieved in 2005.
Guidance provided by the Fresenius Medical Care does not take into effect any expected one-time items and the change of accounting principle for stock options - SFAS 123(R) in the fiscal year 2006. Fresenius Medical Care expects the after tax impact of the one-time items and SFAS 123(R) to be around US$ 50 million.
For further information, please see Fresenius Medical Care's press release at www.fmc-ag.com.
Fresenius Kabi
Fresenius Kabi offers infusion therapies and clinical nutrition for seriously and chronically ill patients in the hospital and out-patient environments. The company is also a leading provider of transfusion technology products.

- Excellent EBIT margin of 13.9 % achieved – guidance exceeded
- Strong organic growth of 7 %
- Outlook 2006: significant growth in sales and earnings expected
Fresenius Kabi's sales rose 13 % to € 1,681 million (2004: € 1,491 million). The company achieved excellent organic growth of 7 %. Acquisitions, primarily Labesfal, contributed 5 % to sales. Divestments had a -1 % effect on sales. Currency translation added 2 % to growth. Constant currency growth of 11 % exceeded the company's earlier guidance.
Sales in Europe (excluding Germany) increased 15 % with acquisitions making a significant contribution. Sales in Germany rose 1 %. Fresenius Kabi continued to grow exceptionally outside of Europe and achieved sales growth of 17 % in Asia-Pacific, 28 % in Latin America and 13 % in Africa.
Fresenius Kabi achieved a new record EBIT with a 33 % increase to € 234 million (2004: € 176 million). The EBIT margin improved by 210 basis points to 13.9 % (2004: 11.8 %). Key drivers were the strong sales growth, further cost optimization and improved efficiency, especially in production.
Fresenius Kabi expects the positive development to continue in 2006. Sales are expected to increase about 10 % in constant currency. The Asia-Pacific and Latin America regions are projected to continue their growth pattern. The first-time consolidation of Clinico and Pharmatel will also have a positive effect on sales. Pharmatel is an Australian company, in which Fresenius Kabi increased its stake from 25.1 % to 50.1 % at the beginning of 2006. The projected sales growth combined with cost optimizations will result in a significant earnings improvement in 2006. Fresenius Kabi's EBIT-margin is projected to increase to 14.5 - 15.0 %.
Fresenius ProServe
Fresenius ProServe offers services for the international health care sector including hospital operations, technical management and hospital planning and construction as well as planning and construction of pharmaceutical and medical-technical production sites.

*incl. HELIOS
- Acquisition of HELIOS Kliniken successfully closed
- 2005 sales and earnings within expectations
- Outlook 2006: Further positive development expected
EBIT was € 20 million (2004: € 9 million; before one-time expenses: € 17 million), in line with the company's expectations.
Order intake and order backlog at the project business developed very positively: Order intake increased 40 % to € 341 million (2004: € 244 million). Order backlog rose 7 % to € 360 million (December 31, 2004: € 335 million).
The acquisition of HELIOS was completed at the end of 2005. The company was consolidated as of December 31, 2005 in the Group's balance sheet.
HELIOS developed positively in 2005 and met its communicated targets. Sales reached € 1,200 million. EBIT was € 105 million, the EBIT margin therefore 8.8 %. Net income amounted to € 67 million. The figures are in accordance with US-GAAP as followed by the Fresenius Group. In 2004, HELIOS had prepared its financial statements according to International Finance Reporting Standards (2004 IFRS: sales € 1,161 million, EBIT € 95 million, net income € 66 million).
Through the acquisition of HELIOS, Fresenius ProServe has become a strong third business segment within the Fresenius Group. Including HELIOS for the full 2005 financial year, sales at Fresenius ProServe were € 2,009 million and EBIT € 125 million.
For 2006, Fresenius ProServe expects an organic sales growth of 1 to 3 % based on 2005 sales of € 2,009 million. Projected EBIT will be between € 140 million and € 150 million.
Video Webcast
As part of the publication of our 2005 results, a press conference will be held on February 22, 2006 at 10:00 a.m. CET. We cordially invite you to follow the live video broadcast of the conference over the Internet at www.fresenius-ag.com. Following the conference, a recording of the conference will be available as video-on-demand.
Annual report
The 2005 Annual Report will be available on March 20, 2006 on the Internet at www.fresenius-ag.com / Investor Relations / Publications.
For tables Fresenius Group in Figures and Consolidated statement of income (US GAAP) see pdf file.



