
Fresenius Medical Care, the world’s largest provider of dialysis products and services, announced today that it has appointed Dr. Frank Maddux as Global Chief Medical Officer. With this newly created position the company further advances the great importance of applying clinical science at an ever-higher level. This includes also the facilitation of enhanced cooperation and exchange of knowledge across the entire Fresenius Medical Care network to ensure high-quality outcomes for patients worldwide.
Rice Powell, Chief Executive Officer of Fresenius Medical Care and Chairman of the Management Board, said: “Each of us understands that the well-being of our patients is our priority, and key to the company’s success all over the world. To continuously deliver on this commitment, it is becoming increasingly important that we also coordinate the interpretation of clinical science and medical practice patterns on a global basis. With Dr. Maddux we have a Global Chief Medical Officer with a well-deserved, excellent reputation inside and outside our company. He will ensure that we utilize the benefits of our global, vertically integrated approach to achieve the best clinical outcomes for our patients, their families and the payor community as well. Our Medical Office led by Dr. Maddux will be key to our ability to drive value for our patients by pursuing new and evolving medical opportunities, such as a more focused home therapies offering, regenerative medicine or enhancing our care coordinated business model throughout the world.”
Dr. Maddux is a physician, IT entrepreneur and health care executive with more than 30 years of health care experience. He has been working for Fresenius Medical Care since 2009. Most recently Dr. Maddux served as Executive Vice President for Clinical & Scientific Affairs and Chief Medical Officer for Fresenius Medical Care North America with responsibility for the delivery of high-quality, value-based care for the largest integrated renal care network in the U.S. His outstanding expertise and research interests have been focused on the quality of care for chronic kidney disease patients.
This release contains forward-looking statements that are subject to various risks and uncertainties. Actual results could differ materially from those described in these forward-looking statements due to various factors, including, but not limited to, changes in business, economic and competitive conditions, legal changes, regulatory approvals, results of clinical studies, foreign exchange rate fluctuations, uncertainties in litigation or investigative proceedings, and the availability of financing. These and other risks and uncertainties are detailed in Fresenius Medical Care AG & Co. KGaA's reports filed with the U.S. Securities and Exchange Commission. Fresenius Medical Care AG & Co. KGaA does not undertake any responsibility to update the forward-looking statements in this release.
Fresenius Kabi announced today that the Committee for Medicinal Products for Human Use (CHMP) of the European Medicines Agency (EMA) has issued a positive opinion, recommending marketing authorization for MSB11022 a biosimilar candidate of Humira® (adalimumab)*. The European Commission (EC) will now decide on the approval of MSB11022 which is expected in Q2/ 2019.
*Humira® is a registered trademark of AbbVie Biotechnology Ltd.

Fresenius Medical Care, the world’s largest provider of dialysis products and services, today announced the launch of its 4008A dialysis machine, which was especially designed to meet the needs of emerging markets. With the launch of the 4008A, the company is aiming to improve accessibility to life-sustaining dialysis treatment for patients in these countries who are living with end-stage renal disease (ESRD).
The 4008A dialysis machine incorporates Fresenius Medical Care’s high therapy standards while minimizing costs for health care systems. It has primarily been deployed in India, with other countries across the Asia-Pacific region to follow. Worldwide, there is an urgent and growing need for patients with ESRD to receive access to dialysis. A systematic review of worldwide access to treatment estimated that almost two million people in Asia with ESRD who needed dialysis were not receiving it – a treatment gap that is double the number of patients actively being treated. Worldwide use of dialysis is projected to more than double by 2030, with the most growth in Asia, but the number of people without access to dialysis is expected to remain substantial.1
In response to this need, Fresenius Medical Care has developed the 4008A, which provides life-sustaining medical benefits and safety standards at a cost-effective price. Dr. Olaf Schermeier, Chief Executive Officer for Global Research and Development at Fresenius Medical Care, emphasized the importance of the 4008A launch: “Our global Research and Development team is in constant dialogue with physicians, nursing staff and patients. We put the benefit for our patients at the center of any new development. That is why we have developed this new high-quality dialysis platform that may allow for better cost effectiveness, and brought it to the market in record time.”
Harry de Wit, CEO of Fresenius Medical Care Asia–Pacific, said: “At Fresenius Medical Care, we are proud of the work we do every day to improve the lives of people living with chronic kidney disease. The launch of the 4008A is a demonstration of our continuing commitment to help ensure that life-changing dialysis is brought within reach of the increasing number of patients who need urgent access to this treatment.”
Importantly, the 4008A offers a high level of safety and handling standards, including essential cleaning functions and battery back-up. The machine is designed to be robust and easily handled, making it ideal for demanding infrastructure and remote locations.
The use of ultrapure dialysis fluid is standard in all treatments with the 4008A machine. Evidence suggests that using ultrapure dialysis fluid provides significant medical benefits to patients – for example, through better preservation of residual renal function, which is the ability of the patient’s own kidneys to eliminate water and uremic toxins.2 This has been shown to lower mortality and potentially improve quality of life in people with ESRD.3,4,5 In addition, the use of ultrapure dialysis fluid could also contribute to reduced inflammation for dialysis patients, potentially helping to reduce their cardiovascular risk and morbidity.6,7
The dialysis machine and the dialyzer are the two most important products in hemodialysis. While the dialyzer filters the patient’s blood, the dialysis machine pumps it and monitors its circulation outside the body. The machine is also used to maintain the composition of the dialysis solution and introduce anticoagulants into the blood. Dialysis treatments generally last three to six hours, and are commonly carried out three times a week.
More than half of all dialysis machines used worldwide are made by Fresenius Medical Care.
References
1 Liyanage T et al. Worldwide access to treatment for end-stage kidney disease: a systematic review. www.thelancet.com Published online March 13, 2015 http://dx.doi.org/10.1016/S0140-6736(14)61601-9.
2 Schiffl H et al. Ultrapure dialysis fluid slows loss of residual renal function in new dialysis patients. Nephrol Dial Transplant 2002;17: 1814–1818.
3 Shafi T., Jaar B., Plantinga L. Association of Residual Urine Output With Mortality, Quality of Life, and Inflammation in Incident Hemodialysis Patients: The Choices for Healthy Outcomes in Caring for End-Stage Renal Disease (CHOICE) Study. Am J Kidney Dis 2010; 56:348-358.
4 Termorshuizen F., Dekker F.Van Manen J. Relative Contribution of Residual Renal Function and Different Measures of Adequacy to Survival in Hemodialysis Patients: An analysis of the Netherlands Cooperative Study on the Adequacy of Dialysis (NECOSAD)-2. J Am Soc Nephrol 2004; 15: 1061–1070.
5 Obi Y., Rhee C., Mathew A. Residual Kidney Function Decline and Mortality in Incident Hemodialysis Patients. J Am Soc Nephrol 2016; 27: 3758–3768.
6 Sitter Tet al. Dialysate related cytokine induction and response to recombinant human erythropoietin in haemodialysis patients.Nephrol Dial Transplant 2000; 15: 2107–1211.
7 Lederer SR, Schiffl H. Ultrapure Dialysis Fluid Lowers the Cardiovascular Morbidity in Patients on Maintenance Hemodialysis by Reducing Continuous Microinflammation. Nephron 2002; 91: 452–455.
Fresenius Medical Care is the world's largest provider of products and services for individuals with renal diseases of which around 3.2 million patients worldwide regularly undergo dialysis treatment. Through its network of 3,872 dialysis clinics, Fresenius Medical Care provides dialysis treatments for 329,085 patients around the globe. Fresenius Medical Care is also the leading provider of dialysis products such as dialysis machines or dialyzers. Along with its core business, the company provides related medical services in the field of Care Coordination. Fresenius Medical Care is listed on the Frankfurt Stock Exchange (FME) and on the New York Stock Exchange (FMS).
For more information visit the Company’s website at www.freseniusmedicalcare.com.
Disclaimer
This release contains forward-looking statements that are subject to various risks and uncertainties. Actual results could differ materially from those described in these forward-looking statements due to certain factors, including changes in business, economic and competitive conditions, regulatory reforms, foreign exchange rate fluctuations, uncertainties in litigation or investigative proceedings, and the availability of financing. These and other risks and uncertainties are detailed in Fresenius Medical Care AG & Co. KGaA's reports filed with the U.S. Securities and Exchange Commission. Fresenius Medical Care AG & Co. KGaA does not undertake any responsibility to update the forward-looking statements in this release.
Unicyte AG, a subsidiary of Fresenius Medical Care, has initiated a long-term research collaboration with Prof. Dr. Tobias B. Huber of Germany’s Medical Center Hamburg-Eppendorf (UKE) to explore the molecular and cellular mechanisms of kidney diseases.
Prof. Huber, Chair of the III. Department of Medicine at UKE, a major university hospital, is a leading expert on glomerular diseases. He will direct the research program, which aims to address various forms of kidney disease including diabetic nephropathy. The goals of the collaboration are to gain further insights into the process leading to the gradual decline in renal function and to identify genetic, signaling and soluble factors involved in the underlying mechanisms.
Unicyte, a leading regenerative medicine company with translational programs in the field of kidney disorders and other illnesses, is building on successful partnerships with other academic partners. These include a collaboration of 15 years’ standing with the University of Torino in Italy.
Dr. Olaf Schermeier, Fresenius Medical Care’s CEO for Global Research and Development, said: “As Unicyte’s organizational structure is designed to leverage the benefits of academia-industry partnerships, the collaboration with Prof. Huber offers a unique opportunity to help patients with kidney disorders by developing innovative treatment options based on a holistic understanding of renal dysfunction. I’m very excited about this collaboration with one of the leading hospitals in Europe, as it could lead to new treatment options that will complement current kidney care.”
Prof. Tobias B. Huber stated: “Because fundamental scientific questions concerning the pathomechanisms of kidney diseases remain to be answered, we will further explore the potential of new options for the treatment of kidney patients in pre-dialysis stages. The collaboration with Unicyte as industry partner will be fruitful in developing a therapy from pre-clinical research through to clinical practice. Our goal is to provide excellent new therapies, reduce the burden of disease, and significantly improve the lives of our patients.”
This release contains forward-looking statements that are subject to various risks and uncertainties. Actual results could differ materially from those described in these forward-looking statements due to certain factors, including changes in business, economic and competitive conditions, regulatory reforms, foreign exchange rate fluctuations, uncertainties in litigation or investigative proceedings, and the availability of financing. These and other risks and uncertainties are detailed in Fresenius Medical Care AG & Co. KGaA's reports filed with the U.S. Securities and Exchange Commission. Fresenius Medical Care AG & Co. KGaA does not undertake any responsibility to update the forward-looking statements in this release.
Fresenius Kabi has received a Drug Shortage Assistance Award from the U.S. Food and Drug Administration (FDA). The FDA recognized the role Fresenius Kabi played in helping mitigate a shortage of IV saline, specifically Sodium Chloride Injection USP, 0.9% in bags. The shortage was caused last year by supply disruptions at manufacturers affected by Hurricane Maria. The hurricane devastated Puerto Rico, where many companies produce medicines for U.S. hospitals.
Fresenius Kabi has signed a worldwide settlement and license agreement with AbbVie, settling all pending patent litigations between the two companies. Under the terms of the agreement and subject to marketing authorization by the health authorities, Fresenius Kabi’s biosimilar candidate of Humira®* (adalimumab), MSB11022**, could be commercialized in the United States from September 30, 2023.
On October 17, 2018 licenses under the agreement came into effect in certain countries in Europe in which AbbVie owns intellectual property. The application for marketing authorization for MSB11022 was submitted by Fresenius Kabi to the European Medicines Agency (EMA) at the end of last year. The dossier is currently under review. A first launch in Europe is expected in the first half of 2019.
“This agreement is a major step on our way to successfully developing and commercializing our biosimilar portfolio," said Dr. Michael Schönhofen, Member of the Fresenius Kabi Management Board and President of the Pharmaceuticals Division. “In line with our caring for life philosophy, our aim is to contribute to broader access to affordable therapies for chronic and acute diseases. Biosimilar drugs are an increasingly important component in reaching this aim – to the benefit of patients and healthcare systems. The agreement with AbbVie provides further clarity regarding when we will be able to commercialize our biosimilar candidate of Humira®.”
*Humira® (adalimumab) is a registered trademark of AbbVie Biotechnology Ltd.
**MSB11022 is being developed as a biosimilar candidate of Humira® and is not yet approved by health authorities.
This release contains forward-looking statements that are subject to various risks and uncertainties. Future results could differ materially from those described in these forwardlooking statements due to certain factors, e.g. changes in business, economic and competitive conditions, regulatory reforms, results of clinical trials, foreign exchange rate fluctuations, uncertainties in litigation or investigative proceedings, and the availability of financing. Fresenius Kabi does not undertake any responsibility to update the forwardlooking statements in this release.
Fresenius Kabi has reached a milestone on the road to approval for another biosimilar. MSB11455, a biosimilar candidate for Neulasta® (pegfilgrastim), has met its primary endpoints in the two pivotal clinical studies. For more information please see the website of Fresenius Kabi.
Fresenius Medical Care, the world’s largest provider of dialysis products and services, supports the recommendation by the United Kingdom’s National Institute for Health and Care Excellence (NICE) to facilitate population healthcare management for UK patients with end-stage renal disease. In a guideline on renal replacement therapy published on October 3, 2018, NICE, a non-departmental public body of the UK’s Department of Health, advises health and social care professionals to consider hemodiafiltration (HDF) rather than high-flux hemodialysis (HD) if the patient is treated in-center, due to the positive impact on patient outcomes and cost-effectiveness.
High-flux HD is the application of high-flux dialyzers to remove a broad spectrum of small and large uremic toxins in order to contribute towards better management of dialysis patients. HDF adds a convective component to the high-flux HD treatment, making it even more effective in removing larger toxins and further improving patient outcomes.
In order to provide evidence-based guidance, NICE systematically reviewed best available renal replacement therapy studies and conducted an economic evaluation. The assessment identified clear benefits of HDF treatment for both renal disease patients and the national healthcare system, as HDF is not only considered cost-effective but is also expected to decrease mortality rates among patients.
Fresenius Medical Care has a long-standing commitment to make HDF available to patients to achieve the best possible outcomes. The company has developed innovative technologies which automatically maximize substitution rates in HDF and support the application of HighVolumeHDF®. This makes HDF treatments as simple as HD, and allows HDF treatments to be delivered at the same cost as HD in the UK.
Dr. Katarzyna Mazur-Hofsaess, Chief Executive Officer of Fresenius Medical Care for Europe, the Middle East and Africa, said: “The NICE guidelines incorporate the existing evidence base for the benefits of HDF therapy. We are convinced that our HighVolumeHDF® therapy platform provides the easiest way for the user to deliver HDF to patients. Our commitment to make HighVolumeHDF® available to all patients across the UK remains a top priority for us.”
This release contains forward-looking statements that are subject to various risks and uncertainties. Actual results could differ materially from those described in these forward-looking statements due to certain factors, including changes in business, economic and competitive conditions, regulatory reforms, foreign exchange rate fluctuations, uncertainties in litigation or investigative proceedings, and the availability of financing. These and other risks and uncertainties are detailed in Fresenius Medical Care AG & Co. KGaA's reports filed with the U.S. Securities and Exchange Commission. Fresenius Medical Care AG & Co. KGaA does not undertake any responsibility to update the forward-looking statements in this release.
Fresenius Medical Care North America is making a $100,000 contribution to the American Nephrology Nurses Association (ANNA), a professional nursing organization with more than 8,500 members. In recognition of ANNA’s 50th anniversary year and Nephrology Nurses Week, the donation will fund an ongoing scholarship program, help increase awareness of the profession, and strengthen engagement with nurses dedicated to caring for patients with chronic kidney disease.
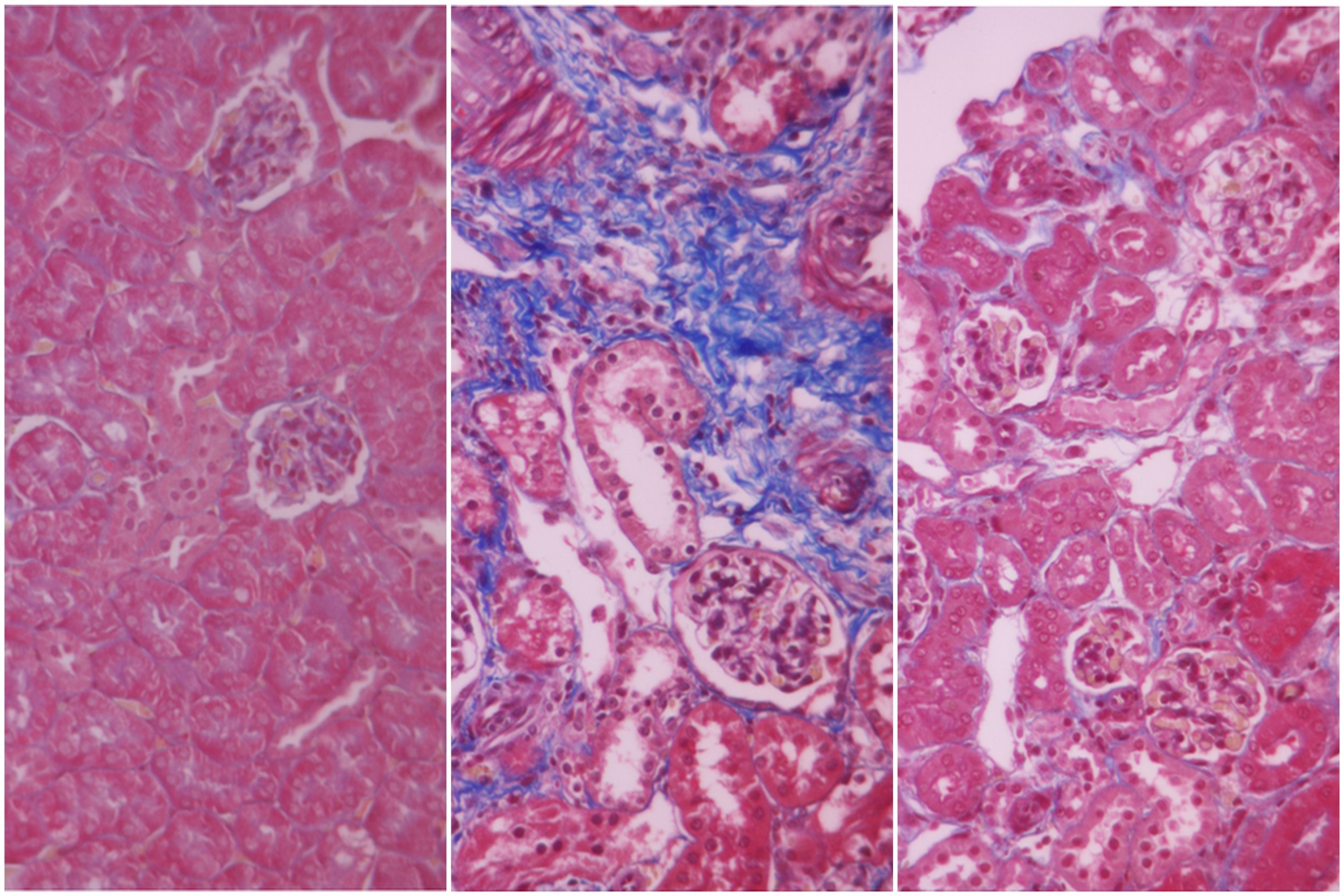
Fresenius Medical Care, the world’s largest provider of dialysis products and services, announced today that its subsidiary Unicyte AG has achieved a key preclinical milestone in its regenerative medicine program for chronic kidney disease. The company was able to confirm a disease modifying potential for its proprietary nano-Extracellular Vesicles (“nEVs” are stem cell-derived particles that support communication between cells) in a second preclinical model of chronic kidney disease.
When administered to mice with fast progressing kidney disease, Unicyte’s nEVs prevented renal fibrosis, a hallmark of chronic kidney disease. In particular, nEVs significantly reduced interstitial fibrosis and tubular necrosis while also inhibiting infiltration of various cells. This resulted in near-to-normal recovery of kidney function. The study conducted in collaboration with Prof. Giovanni Camussi of the University of Turin, Italy, has been accepted for publication in the peer-reviewed journal Frontiers of Immunology (https://doi.org/10.3389/fimmu.2018.01639).
These new results support previous findings in a preclinical model of slowly progressing kidney disease (diabetic nephropathy), a major pathology that often leads to end-stage renal disease. Combined results of these studies demonstrate the efficacy and the underlying mechanism of action of nEVs in preventing renal fibrosis and subsequent progression to end-stage renal disease. Unicyte will continue the preclinical and clinical development of its proprietary nEVs for treatment of chronic and acute kidney diseases.
Dr. Olaf Schermeier, Fresenius Medical Care’s CEO for Global Research and Development, said: “We are very excited about the progress we have made with our research and development activities over the last 30 months since we have established Unicyte. Based on these achievements, Unicyte will continue to explore the potential of nEVs for the treatment of patients in pre-dialysis stages of chronic kidney disease.”
Prof. Giovanni Camussi, Professor Emeritus at the University of Turin and Member of Unicyte’s Scientific Advisory Board, said, “nEVs are a promising regenerative medicine technology platform. Our aim is to develop new and better treatment options for severely and chronically ill patients over time. Achieving this preclinical milestone represents an important step towards testing nEVs in the clinical setting.”
With multiple therapeutic programs at the clinical and preclinical stage, Unicyte has established a broad pipeline in kidney and liver diseases, diabetes and oncology. The company is seeking strategic partnerships for its non-renal programs.
Pagination
- Previous page
- Page 12
- Next page


